Clinical Characteristics of Chronic Myeloid Leukemia Patients with Deletion and Non-deletion of ASS Gene on Derivative Chromosome 9
-
摘要:目的
探讨慢性髓性白血病(CML)慢性期衍生9号染色体ASS基因缺失与非缺失患者的临床特征及疗效。
方法分析初始治疗方案为伊马替尼并采用BCR/ABL1/ASS1 3色融合探针检测ASS基因是否缺失的CML患者的临床资料,分为缺失组(n=27)和非缺失组(n=92),分析其临床特征、治疗效果及预后。
结果119例患者平均年龄37.22±12.72岁,缺失组和非缺失组患者的sokal评分差异有统计学意义(χ2=4.304, P=0.038),其他一般特征差异无统计学意义(P > 0.05)。缺失组的3个月完全细胞遗传学反应(CCyR)率及6个月CCyR率、BCR-ABLIS≤ 1%率均低于非缺失组(均P < 0.05)。随访中位数为35.0(3.0~60.0)个月,缺失组PFS低于非缺失组(χ2=4.293, P=0.038),两组OS比较差异无统计学意义(χ2=0.008, P=0.931)。
结论伊马替尼治疗的CML慢性期患者中ASS基因缺失导致治疗疗效不佳及预后不良,且更易出现疾病进展。
Abstract:ObjectiveTo investigate the clinical characteristics of patients with chronic myeloid leukemia (CML) in chronic phase with deletion and non-deletion of the argininosuccinate synthesis gene (ASS gene) on the derivative chromosome 9.
MethodsThe clinical data of patients with CML initially treated with imatinib and BCR/ABL1/ASS1 3-color fusion probe to detect ASS gene deletion were analyzed. The patients were divided into deletion group (n=27) and non-deletion group (n=92). Clinical characteristics, treatment effects, and prognosis were analyzed.
ResultsThe average age of 119 patients was 37.22±12.72 years old. The sokal score differed between the deletion and non-deletion groups (χ2=4.304, P=0.038). No statistically significant difference in other general characteristics was found (P > 0.05). The 3-month CCyR rate, 6-month CCyR rate, and BCR-ABLIS≤ 1% rate in the deletion group were lower than those in the non-deletion group (P < 0.05). The median follow-up of 119 patients was 35.0 (3.0-60.0) months. The PFS in the deletion group was lower than that in the non-deletion group (χ2=4.293, P=0.038). Overall survival was not significantly different between the two groups (χ2=0.008, P=0.931).
ConclusionThe deletion of the ASS gene in patients with chronic CML is related to the poor efficacy of imatinib treatment, poor prognosis, and high risk of disease progression.
-
0 引言
慢性髓性白血病(chronic myeloid leukemia, CML)是一种起源于多能造血干细胞的克隆性恶性白血病,其细胞遗传学特征是9号和22号染色体的相互易位即Ph染色体[1],其本质是t(9;22)(q34;q11)易位,形成BCR/ABL融合基因,这是CML发病的分子机制。ASS基因是精氨基琥珀酸合成酶的编码基因,称为精氨基琥珀酸合成基因(argininosuccinate synthesis gene, ASS),位于染色体9q34.1,约10%~15%的CML患者在衍生9号染色体[der(9)]易位断裂点附近有较大的序列缺失,伴有该缺失的患者表现出较快的疾病进展及较短的生存期[2-4]。受目前国内ASS基因的检测费用贵、检出率低等因素影响,ASS基因缺失的临床研究相对较少。为了解ASS基因缺失CML患者的临床特征及疗效,本研究通过应用BCR/ABL1/ASS1 3色融合探针荧光原位杂交技术(FISH)方法对CML慢性期患者进行检测,随访并收集临床资料,对比分析ASS基因缺失与非缺失患者的疗效,评估ASS基因缺失在CML预后中可能的作用,为临床提供参考。
1 资料与方法
1.1 病例资料
回顾性分析2014年1月—2021年1月在广州市红十字会医院及南方医科大学南方医院初诊的CML慢性期患者临床资料。纳入标准:(1)符合中国慢性髓性白血病诊断与治疗指南(2016版)[5]相关诊断标准;(2)年龄大于18岁;(3)进行过ASS基因检测及初始治疗方案为伊马替尼靶向治疗。排除标准:(1)诊断时CML分期为加速期及急变期;(2)初治一线方案非伊马替尼治疗。共纳入119例CML患者,其中ASS基因缺失者27例及非缺失者92例。
1.2 研究方法
1.2.1 临床数据资料收集及分组
所有患者均完善骨髓、染色体、基因遗传学及ASS基因检查,明确诊断为CML慢性期。随访截止时间为2021年1月31日或患者死亡。收集患者确诊当次住院的一般资料及实验室检查结果作为基线资料,包括性别、年龄、血常规、血生化、腹部B型超声、骨髓结果、染色体、BCR-ABL FISH及基因定量结果(初诊、3个月、6个月、12个月、移植前、移植后评估及定期随访时间点)[6]。分组:根据ASS基因是否缺失分为:缺失组(n=27)和非缺失组(n=92)。
1.2.2 细胞遗传学分析
采集CML患者初诊时的骨髓,用直接法及短期培养法培养细胞,常规收获细胞并制备染色体。所有标本均采用BCR/ABL1/ASS1 3色融合探针对骨髓间期细胞进行荧光原位杂交,所有探针均购自美国Vysis公司。检测方法按Vysis BCR/ABL1/ASS1 3色DF FISH探针(Vysis,abbott)说明书要求进行检测。
1.2.3 疗效标准
参照2020年美国癌症综合网络(NCCN)[6]发布的CML临床实践指南评估患者的疗效,指标包括完全血液学反应(complete hematologic response, CHR)、完全细胞遗传学反应(complete cytogenetic response, CCyR)、伊马替尼治疗3个月时BCR-ABLIS≤ 10%率、6个月时BCR-ABLIS≤ 1%率及12个月时BCR-ABLIS≤ 0.1% 率。总生存(OS)时间指患者诊断明确至任何原因引起死亡的时间,无疾病进展生存(PFS)指诊断之日至疾病进入加速期或急变期的时间。
1.3 统计学方法
所有数据采用SPSS25.0软件进行统计分析,计数资料用例(%)表示,两者比较采用χ2检验或Fisher精确检验;符合正态分布的计量资料用均数±标准差(x±s)表示,组间均数比较采用独立样本t检验,非正态分布的计量资料用中位数和四分位数(M(P25, P75))表示,组间比较采用Mann-Whitney U检验。生存曲线采用Kaplan-Meier法分析,采用Log rank检验。P < 0.05为差异有统计学意义。
2 结果
2.1 患者一般特征
119例患者中男性80例,女性39例,年龄18~70岁,平均年龄37.22±12.72岁,81例(68.1%)患者年龄大于30岁。白细胞计数中位数为146.82(67.00, 238.90)×109/L,血红蛋白中位数为98.0(83.0, 115.0)g/L,血小板计数中位数为382.0(223.0, 680.0)×109/L,Sokal评分高危组33例(27.7%)、中危组44例(37.0%)、低危组42例(35.3%),5例(缺失组2例、非缺失组3例)患者有附加染色体畸变。
2.2 两组临床基线资料
缺失组和非缺失组患者除了Sokal评分存在差异外,年龄、疾病分期、白细胞计数、血红蛋白、嗜酸性粒细胞计数(EOS)、嗜碱性粒细胞计数(BAS)及血小板等一般特征差异无统计学意义(均P > 0.05),见表 1。
表 1 ASS基因缺失组和非缺失组CML患者临床基线资料比较Table 1 Comparison of clinical baseline date of patients with CML in ASS gene deletion and non-deletion groups
2.3 血液学及遗传学缓解情况
119例CML患者中,118例(99.2%)治疗后获得CHR。缺失组27例患者中,3个月疗效评价均获得CHR。非缺失组92例患者中3个月疗效评价91例(98.9%)获得CHR。两组患者各时点遗传学反应疗效比较,非缺失组在3个月及6个月CCyR率、BCR-ABLIS≤1%率明显高于缺失组,差异有统计学意义(P < 0.05),其余各时点疗效评价两组差异均无统计学意义,见表 2。
表 2 ASS基因缺失组和非缺失组CML患者各时点遗传学反应疗效比较Table 2 Comparison of therapeutic effects of genetic responses among patients with CML in ASS gene deletion and nondeletion groups at each time point
2.4 疾病进展及生存情况
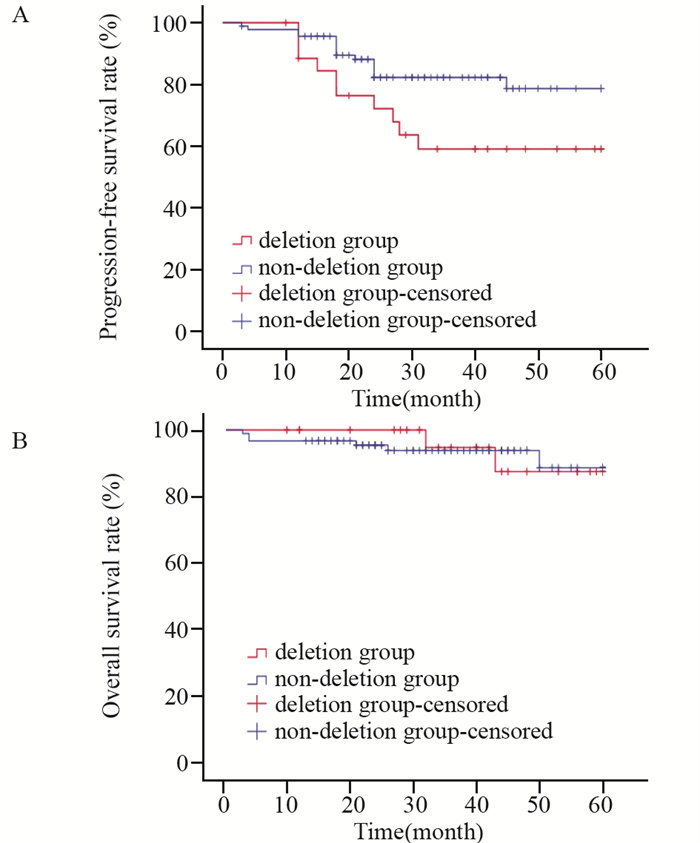
截至随访日期,119例患者随访中位时间为35.0(3.0~60.0)个月,25例(21.0%)出现疾病进展,其中缺失组有10例(37.0%)出现疾病进展,8例改用2代TKI药物(达沙替尼或尼洛替尼)、2例行异基因造血干细胞移植进行后续治疗。非缺失组有15例(16.3%)在治疗过程出现疾病进展,13例改用2代TKI药物(达沙替尼或尼洛替尼),2例行异基因造血干细胞移植进行后续治疗。缺失组和非缺失组之间PFS比较差异有统计学意义(χ2=4.293, P=0.038),见图 1A。119例患者中,8例(6.7%)在随访中死亡,其中缺失组2例(7.4%)(因疾病进展死亡),非缺失组6例(6.5%)(因疾病进展、肺炎和脑出血死亡各2例),缺失组和非缺失组之间OS比较差异无统计学意义(χ2=0.008, P=0.931),见图 1B。
3 讨论
CML的细胞遗传学特征是9号和22号染色体的相互易位,即Ph染色体。目前认为染色体核型的演变与病情进展及TKI药物疗效有关,是预后不良因素[7]。研究报道利用荧光原位杂交技术可在CML患者中检测出衍生9号染色体(der(9))断裂位点附近部分片段缺失[8],derder(9)断裂位点附近片段包含ASS基因片段,研究发现约有16.7%的CML患者存在衍生9号染色体ASS基因缺失[9],有研究报道derder(9)断裂位点附近片段缺失是急慢性白血病不良预后的因素之一[4, 10-11]。本研究结果显示,缺失组Sokal评分中高危组患者比例明显高于非缺失组,说明ASS基因缺失CML患者有更差的预后。目前很多因素影响CML患者产生TKI耐药及疾病进展,其中染色体易位伴随序列缺失、倒位和重排等基因组异常发挥着重要作用[12],Chandran等[2]研究也发现derder(9)断裂位点片段缺失可能与Ph染色体易位同时发生,其发生率在疾病晚期更高。本研究中ASS基因缺失CML患者中只有2例患者染色体为典型Ph+染色体伴其他核型表达,复杂染色体发生率较低,推测可能因为纳入患者数量较少且为初发慢性期有关,需要进一步增加病例数检测ASS基因缺失,并进一步随访检测染色体的状况分析两者之间的关系。
NCCN及国内指南均推荐伊马替尼为CML一线药物且有较好的疗效[13-14]。Gorusu等[15]研究发现derder(9)断裂位点片段缺失CML患者在伊马替尼之后的血液学和细胞遗传学反应均较低,在治疗1年后表现出比没有缺失患者更差的疗效,国内有关学者[16]也报了5例ASS缺失CML使用TKI药物治疗后仅有1例获得分子遗传学缓解。本研究分析了缺失组和非缺失组两组患者伊马替尼治疗各时点遗传学反应疗效,结果表明,缺失组在3个月CCyR率、6个月CCyR率及BCR-ABLIS≤1%率均明显低于非缺失组,也进一步说明ASS基因缺失影响伊马替尼对CML的疗效。而有关学者在相关研究中derder(9)断裂位点片段缺失并不影响伊马替尼治疗CML的疗效,在细胞遗传学反应及总生存方面与对照组相比较并没有明显差异,他们建议对于derder(9)缺失CML患者可以考虑以同样的方式接受伊马替尼的治疗[17-20]。本研究ASS基因缺失患者接受伊马替尼治疗后,12个月进行疗效评价显示,虽然缺失组疗效反应率低于非缺失组,但差异无统计学意义,需进一步延长随访时间评估两组的疗效。
另有研究表明derder(9)断裂位点片段缺失对P53和P73的激活产生负面影响,并使肿瘤细胞通过TGF-β信号通路侵入正常组织,致使患者较快发生疾病进展[21],还可能与Ph染色体变异型易位的形成机制有关[22]。有研究[16, 23]发现对于derder(9)断裂位点片段缺失患者,无论是慢性期或进展期更易出现疾病进展。本研究中,与非缺失组相比较,有37.0%的ASS基因缺失CML患者出现疾病进展,且两组患者PFS差异有明显统计学意义,提示ASS基因缺失对CML疾病进展有显著影响,与相关学者[2]报道类似。和传统的Sokal、Hasford评分系统一样,ASS基因缺失也可能成为评估CML患者预后的因素之一。虽然缺失组部分患者选择异基因造血干细胞移植,但两组总生存率差异无明显统计学意义,推测原因可能与我们在发现伊马替尼耐药后或疾病进展时及时调整TKI药物及随访时间有限有关。我们进一步随访发现疾病进展CML患者改用二代TKI药物治疗后,均有明显疗效,李珍等[24]研究发现伊马替尼联合三氧化二砷对ASS基因缺失CML患者有较好的疗效,说明监测疾病进展并及时更改治疗方案,可改善ASS基因缺失患者预后。
综上所述,CML患者中ASS基因缺失与伊马替尼治疗疗效及不良预后有关,且更易出现疾病进展,需在治疗过程中密切监测治疗反应。由于本研究患者的样本量少,随访时间有限,研究存在一定的局限性,ASS基因缺失的价值需扩大样本量进一步研究证实。
Competing interests: The authors declare that they have no competing interests.作者贡献:高冠论、周璇:课题设计、资料分析、撰写论文许娜、魏婷:数据统计分析与修改核对刘晓力、李庆山:拟定写作思路、指导论文撰写与修改 -
表 1 ASS基因缺失组和非缺失组CML患者临床基线资料比较
Table 1 Comparison of clinical baseline date of patients with CML in ASS gene deletion and non-deletion groups

表 2 ASS基因缺失组和非缺失组CML患者各时点遗传学反应疗效比较
Table 2 Comparison of therapeutic effects of genetic responses among patients with CML in ASS gene deletion and nondeletion groups at each time point

-
[1] Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring[J]. Am J Hematol, 2020, 95(6): 691-709. doi: 10.1002/ajh.25792
[2] Chandran RK, Geetha N, Sakthivel KM, et al. Prognostic Implications of Derivative Chromosome 9 Deletions in Patients with Advanced-Stage Chronic Myelogenous Leukemia[J]. J Environ Pathol Toxicol Oncol, 2018, 37(2): 117-126. doi: 10.1615/JEnvironPatholToxicolOncol.2018026023
[3] Asnafi AA, Deris Zayeri Z, Shahrabi S, et al. Chronic myeloid leukemia with complex karyotypes: Prognosis and therapeutic approaches[J]. J Cell Physiol, 2019, 234(5): 5798-5806. doi: 10.1002/jcp.27505
[4] Li JY, Xu W, Wu W, et al. The negative prognostic impact of derivative 9 deletions in patients who received hydroxyurea treatment for chronic myelogenous leukemia in the chronic phase[J]. Onkologie, 2008, 31(11): 585-589.
[5] 中华医学会血液学分会. 中国慢性髓性白血病诊断与治疗指南(2016年版)[J]. 中华血液学杂志, 2016, 37(8): 633-639. https://xuewen.cnki.net/CCND-YIYA201610190061.html Chinese Society of Hematology, Chinese Medical Association. The guidelines for diagnosis and treatment of chronic myelogenous leukemia in China (2016 edition)[J]. Zhonghua Xue Ye Xue Za Zhi, 2016, 37(8): 633-639. https://xuewen.cnki.net/CCND-YIYA201610190061.html
[6] Deininger MW, Shah NP, Altman JK, et al. Chronic Myeloid Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2020, 18(10): 1385-1415. doi: 10.6004/jnccn.2020.0047
[7] Zhou T, Medeiros LJ, Hu S. Chronic Myeloid Leukemia: Beyond BCR-ABL1[J]. Curr Hematol Malig Rep, 2018, 13(6): 435-445. doi: 10.1007/s11899-018-0474-6
[8] Zhang H, Liu M, Wang X, et al. Genomic Copy Number Variants in CML Patients With the Philadelphia Chromosome (Ph+): AnUpdate[J]. Front Genet, 2021, 12: 697009. doi: 10.3389/fgene.2021.697009
[9] 张朕豪, 王艳芳, 王淼, 等. 应用三色双融合探针检测BCR-ABL融合基因及ASS1基因缺失[J]. 中国实验血液学杂志, 2020, 28(4): 1115-1122. doi: 10.19746/j.cnki.issn1009-2137.2020.04.006 Zhang ZH, Wang YF, Wang M, et al. [Detection of BCR/ABL Fusion Gene and ASS1 Gene Deletion by Using Tricolor Dual-fusion Probe[J]. Zhongguo Shi Yan Xue Ye Xue Za Zhi, 2020, 28(4): 1115-1122. doi: 10.19746/j.cnki.issn1009-2137.2020.04.006
[10] Fourouclas N, Campbell PJ, Bench AJ, et al. Size matters: the prognostic implications of large and small deletions of the derivative 9 chromosome in chronic myeloid leukemia[J]. Haematologica, 2006, 91(7): 952-955
[11] Egan D, Radich J. Making the diagnosis, the tools, and risk stratification: More than just BCR-ABL[J]. Best Pract Res Clin Haematol, 2016, 29(3): 252-263. doi: 10.1016/j.beha.2016.10.015
[12] Fernandes A, Shanmuganathan N, Branford S. Genomic Mechanisms Influencing Outcome in Chronic Myeloid Leukemia[J]. Cancers (Basel), 2022, 14(3): 620. doi: 10.3390/cancers14030620
[13] Hochhaus A, Baccarani M, Silver RT, et al. European leukemianet 2020 recommendations for treating chronic myeloid leukemia[J]. Leukemia, 2020, 34: 966-984. doi: 10.1038/s41375-020-0776-2
[14] Hochhaus A, Larson RA, Guilhot F, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia[J]. N Engl J Med, 2017, 376(10): 917-927. doi: 10.1056/NEJMoa1609324
[15] Gorusu M, Benn P, Li Z, et al. On the genesis and prognosis of variant translocations in chronic myeloid leukemia[J]. Cancer Genet Cytogenet, 2007, 173(2): 97-106. doi: 10.1016/j.cancergencyto.2006.10.006
[16] 董洁, 李薇, 白晶, 等. 9号衍生染色体在慢性粒细胞白血病预后评估中的意义[J]. 吉林大学学报(医学版), 2016, 42(2): 301-305. https://www.cnki.com.cn/Article/CJFDTOTAL-BQEB201602021.htm Dong J, Li W, Bai J, et al. Significance of derivative chromosome 9 in evaluation on prognosis of chronic myeloid leukemia[J]. Jilin Da Xue Xue Bao(Yi Xue Ban), 2016, 42(2): 301-305. https://www.cnki.com.cn/Article/CJFDTOTAL-BQEB201602021.htm
[17] Kim DH, Popradi G, Sriharsha L, et al. No significance of derivative chromosome 9 deletion on the clearance kinetics of BCR/ABL fusion transcripts, cytogenetic or molecular response, loss of response, or treatment failure to imatinib mesylate therapy for chronic myeloid leukemia[J]. Cancer, 2008, 113(4): 772-781. doi: 10.1002/cncr.23607
[18] Švabek ŽT, Josipović M, Horvat I, et al. The incidence of atypical patterns of BCR-ABL1 rearrangement and molecular-cytogenetic response to tyrosine kinase inhibitor therapy in newly diagnosed cases with chronic myeloid leukemia (CML)[J]. Blood Res, 2018, 53(2): 152-159. doi: 10.5045/br.2018.53.2.152
[19] Quintás-Cardama A, Kantarjian H, Shan J, et al. Prognostic impact of deletions of derivative chromosome 9 in patients with chronic myelogenous leukemia treated with nilotinib or dasatinib[J]. Cancer, 2011, 117(22): 5085-5093. doi: 10.1002/cncr.26147
[20] Bennour A, Ouahchi I, Ben Youssef Y, et al. Molecular cytogenetic study of derivative chromosome 9 deletion in chronic myeloid leukemia patients[J]. Med Oncol, 2012, 29(2): 1151-1160. doi: 10.1007/s12032-011-9918-8
[21] Jiang Y, Zhang J, Guo D, et al. Entire ABL1 Gene Deletion Without BCR/ABL1 Rearrangement in a Female Patient with B-Cell Precursor Acute Lymphoblastic Leukemia[J]. Onco Targets Ther, 2020, 13: 783-790. doi: 10.2147/OTT.S238336
[22] Bennour A, Sennana H, Laatiri MA, et al. Molecular cytogenetic characterization of variant Philadelphia translocations in chronic myeloid leukemia: genesis and deletion of derivative chromosome 9[J]. Cancer Genet Cytogenet, 2009, 194(1): 30-37. doi: 10.1016/j.cancergencyto.2009.05.010
[23] Kreil S, Pfirrmann M, Haferlach C, et al. Heterogeneous prognostic impact of derivative chromosome 9 deletions in chronic myelogenous leukemia[J]. Blood, 2007, 110(4): 283-1290.
[24] 李珍, 张龑莉, 赵慧芳, 等. 伊马替尼联合三氧化二砷治疗伴有ASS基因缺失的Ph+慢性粒细胞白血病的临床研究[J]. 中国实用医刊, 2019, 46(11): 97-99. https://www.cnki.com.cn/Article/CJFDTOTAL-HBZY201812008.htm Li Z, Zhang YL, Zhao HF, et al. Effects of imatinib combined arsenic trioxide on Ph+chronic myeloid leukemia patients lacking of ASS genes[J]. Zhongguo Shi Yong Yi Kan, 2019, 46(11): 97-99. https://www.cnki.com.cn/Article/CJFDTOTAL-HBZY201812008.htm



 下载:
下载: