Clinical Application of A New In Vitro High-throughput Drug Sensitivity Screening Technology in Individualized Medication of Relapsed Refractory Acute Myeloid Leukemia Patients
-
摘要:目的
利用新型高通量药敏检测技术(HDS)探讨复发难治性急性髓系白血病患者的个体化治疗。
方法获取19例复发难治性急性髓系白血病患者的骨髓样本,HDS技术对患者自体肿瘤白细胞进行富集培养,对15种单药和17种化疗联合方案按照临床剂量对应的血液峰浓度进行体外药效评估,化学发光法(Celltiter-Glo)行细胞活性检测并计算抑制率,根据抑制率判断药物/方案的敏感等级,为临床制定用药方案提供参考并观察临床缓解率。
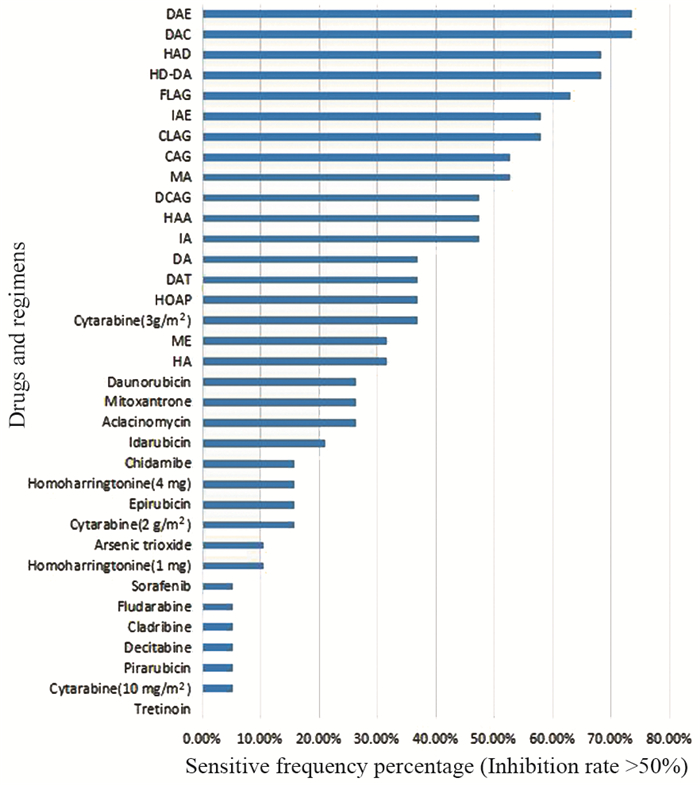
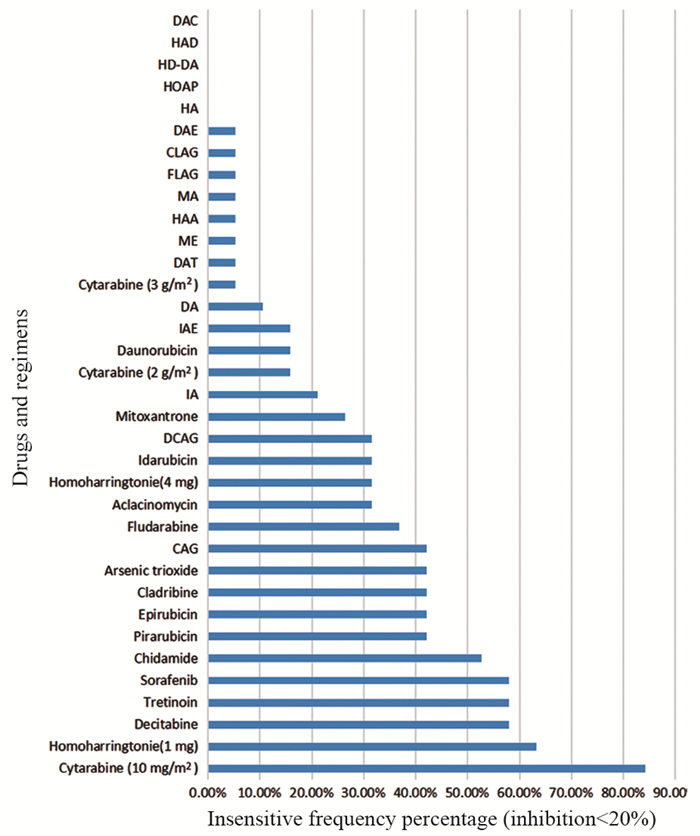
结果19例患者中,4例未缓解(NR)、4例完全缓解(CR)、11例部分缓解(PR),总缓解率(ORR)为78.94%。DAE、DAC、HAD、HD-DA化疗方案敏感频次在68%以上。
结论HDS在急性白血病个体化用药领域有很好的临床应用价值,是后基因组时代值得临床推广的一种快速、高效、低成本的检测技术手段。
Abstract:ObjectiveTo explore the clinical value of new high-throughput drug sensitivity (HDS) screening technology in the individualized medication of relapsed refractory acute myeloid leukemia patients.
MethodsWe collected bone marrow samples of 19 patients with relapsed and refractory acute myeloid leukemia, enriching and culturing leukemic cancer cells by HDS technology. In vitro efficacy evaluation of 15 single drugs and 17 chemotherapy regimens was carried out according to the blood peak concentration corresponding to clinical dose. The drugs and programs were divided into four levels: high, medium, low and insensitive, according to the inhibition rate of cell activity detected and calculated by Celltiter-Glo based chemiluminescence, then we observed the clinical remission rate.
ResultsAmong the 19 patients, there were 4 cases of non-remission (NR), 4 cases of complete remission (CR) and 11cases of partial remission (PR), and the overall remission rate was 78.94%. The sensitivity frequency of DAE, DAC, HAD and HD-DA schemes were higher than 68%.
ConclusionThe high-throughput drug sensitivity screening technology is a rapid, efficient and low-cost detection technology, with good clinical application value in individualized medication of relapsed refractory AML patients, worthy of clinical promotion in the post genomic era.
-
Key words:
- Drug sensitivity /
- AML /
- High-throughput /
- HDS
-
作者贡献吴海兵:标本搜集、协助临床药板制定及文章撰写赵晓燕、沈明芳:收集病例资料、随访黄瑞:药敏数据分析、文献查阅陈程:原代细胞培养任涛:高通量药物药板剂量设置和药板制定沈旸:项目总负责、质量控制
-
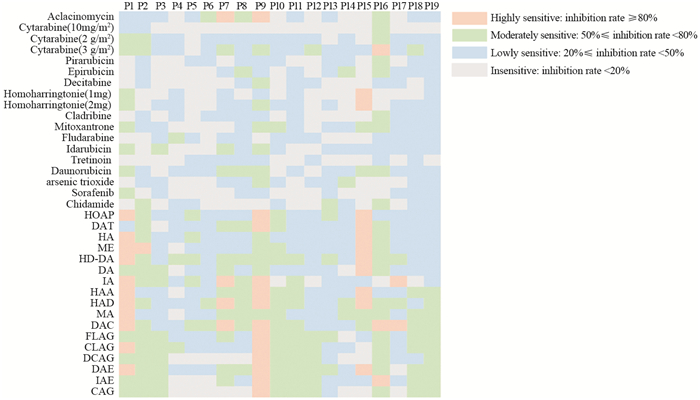
表 1 19例AML患者临床特征和药敏结果
Table 1 Clinical features and drug sensitivity results of 19 AML patients

-
[1] Garnett MJ, Edelman EJ, Heidorn SJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells[J]. Nature, 2012, 483(7391): 570-575. doi: 10.1038/nature11005
[2] Pemovska T, Kontro M, Yadav B, et al. Individualized Systems Medicine Strategy to Tailor Treatments for Patients with Chemorefractory Acute Myeloid Leukemia[J]. Cancer Discov, 2013, 3(12): 1416-1429. doi: 10.1158/2159-8290.CD-13-0350
[3] Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer[J]. Science, 2014, 346(6216): 1480-1486. doi: 10.1126/science.1254721
[4] Chia S, Low JL, Zhang X, et al. Phenotype-driven precision oncology as a guide for clinical decisions one patient at a time[J]. Nat Commun, 2017, 8(1): 435. doi: 10.1038/s41467-017-00451-5
[5] Tyner JW, Tognon CE, Bottomly D, et al. Functional genomic landscape of acute myeloid leukaemia[J]. Nature, 2018, 562(7728): 526-531. doi: 10.1038/s41586-018-0623-z
[6] Pemovska T, Kontro M, Yadav B, Edgren H, et al. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia[J]. Cancer Discov, 2013, 3(12): 1416-1429. doi: 10.1158/2159-8290.CD-13-0350
[7] Creutzig U, Kaspers GJ. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia[J]. J Clin Oncol, 2004, 22(16): 3432-3433. doi: 10.1200/JCO.2004.99.116
[8] Marquart J, Chen EY, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Benefit From Genome-Driven Oncology[J]. JAMA Oncol, 2018, 4(8): 1093-1098. doi: 10.1001/jamaoncol.2018.1660
[9] Jiang Y, Sun A, Zhao Y, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma[J]. Nature, 2019, 567(7747): 257-261. doi: 10.1038/s41586-019-0987-8
[10] Friedman AA, Letai A, Fisher DE, et al. Precision medicine for cancer with next-generation functional diagnostics[J]. Nat Rev Cancer, 2015, 15(12): 747-756. doi: 10.1038/nrc4015
[11] Yan X, Zhou L, Wu Z, et al. High throughput scaffold-based 3D micro-tumor array for efficient drug screening and chemosensitivity testing[J]. Biomaterials, 2019, 198: 167-179. doi: 10.1016/j.biomaterials.2018.05.020
[12] 朱路, 勾越阳, 鄢晓君, 等.基于病人自体肿瘤细胞体外培养模型的个体化精准用药[J].中国医疗设备, 2016, 31(6): 13-18, 35. doi: 10.3969/j.issn.1674-1633.2016.06.003 Zhu L, Gou YY, Yan XJ, et al. Precise medicine in cancer treatmentvia patient derivedin vitro tumor model[J]. Zhongguo Yi Liao She Bei, 2016, 31(6): 13-18, 35. doi: 10.3969/j.issn.1674-1633.2016.06.003
[13] 刘飞扬, 黄瑞, 任涛, 等.肿瘤精准用药治疗的技术体系[J].高科技与产业化, 2016, 242: 50-52. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gkjycyh201607007 Liu FY, Huang R, Ren T, et al. Technology system of cancer Precision medicine[J]. Gao Ke Ji Yu Chan Ye Hua, 2016, 242: 50-52. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gkjycyh201607007
[14] Liu X, Ory V, Chapman S, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells[J]. Am J Pathol, 2012, 180(2): 599-607. doi: 10.1016/j.ajpath.2011.10.036
[15] Almosailleakh M, Schwaller J. Murine Models of Acute Myeloid Leukaemia[J]. Int J Mol Sci, 2019, 20(2). pii: E453. doi: 10.3390/ijms20020453
[16] Mazzocchi AR, Rajan SAP, Votanopoulos KI, et al. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening[J]. Sci Rep, 2018, 8(1): 2886. doi: 10.1038/s41598-018-21200-8



 下载:
下载: