Mediastinal and Left Lung Malignancy Coupling with Suspicion for Esophagus Cancer: A Case Report
-
-
关键词:
- 肺癌 /
- 食管癌 /
- 间变性淋巴瘤激酶(ALK) /
- 克唑替尼
-
0 引言
在肿瘤诊断过程中,对于病理无法明确诊断的患者,尤其是以转移性病灶为首发表现时,我们需充分借助影像学检查及结合肿瘤自身特点协助诊断。四川大学华西医院最近收治纵隔及左肺恶性肿瘤伴食管癌待排患者1例,现报道如下。
1 病例资料
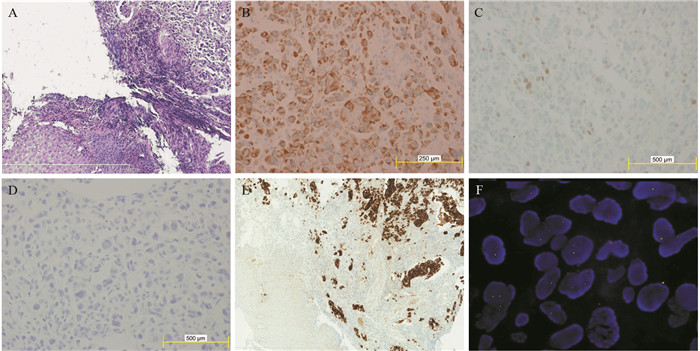
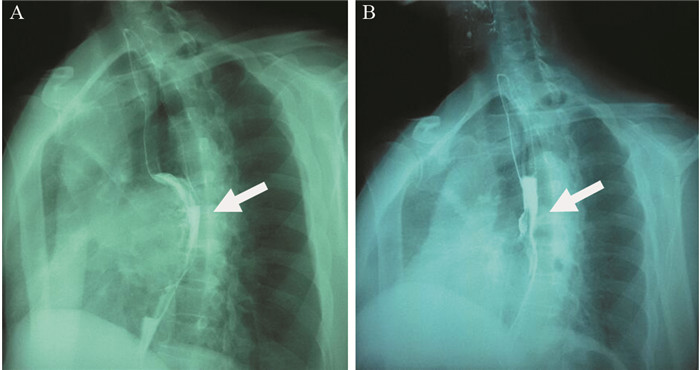
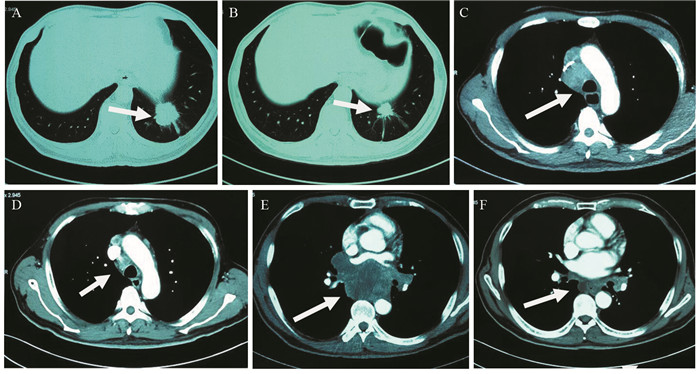
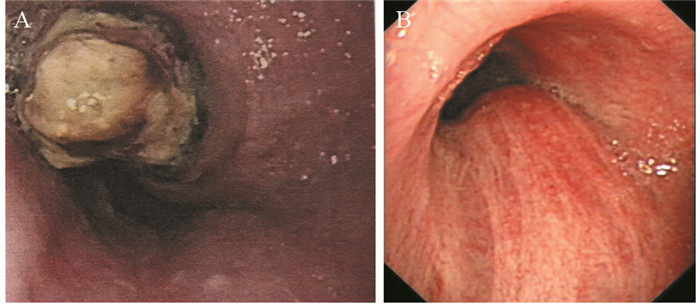
患者57岁,男性,因“进行性吞咽梗阻2月余,咳嗽伴痰中带血一周”于2015年7月16日入院。入院前2月余患者进食干硬食物时感吞咽梗阻。2月以来吞咽梗阻感进行性加重,并逐渐出现咳嗽、咯痰、痰中带血。胃镜提示:食管距门齿27 cm处可见巨大结节样新生物,见图 1A。纤支镜示:左主支气管上段内后壁外压隆起,未见新生物,见图 1B。病理学示:食管内恶性肿瘤倾向低分化癌,见图 2A。免疫组织化学染色:PCK(+)、CK7(+)、TTF-1(+)、CK5/6(-)、P63(-)、CD56(-)、Syn(-)、CgA(-)、NapsinA(-),支持低分化腺癌诊断,但不能确定为肺来源,见图 2A~2D。ALK经免疫组织化学ALK-V及FISH检测(ALK-FISH)均为阳性,见图 2E~2F。食管造影:食管中下段狭窄、管壁僵硬、黏膜破坏,病变长约10.5 cm,见图 3A。胸部增强CT见左肺下叶后基底段约3.7 cm×3.6 cm肿块影,呈分叶状,见毛刺征;纵隔及双肺门淋巴结肿大融合呈团块,最大者短径约3.0 cm;后纵隔约10.1 cm×5.8 cm肿块影,压迫食管,与食管壁分界欠清,见图 4A、4C、4E。头部MRI提示颅内转移瘤。上腹部CT、全身骨显像未见肿瘤转移征象。CEA 31.87 ng/ml,CYFRA21-1 15.49 ng/ml。颈部彩超提示右侧锁骨上区肿大淋巴结,较大约19 mm ×15 mm。入院前2月,体重减轻约20公斤。嗜烟30余年,每天10支。
![]() 图 1 纵隔及左肺恶性肿瘤伴食管癌待排患者胃镜及纤维支气管镜图像Figure 1 Gastroscopy and fibro bronchoscope images of mediastinal and left lung malignancy coupling with suspicion for esophagus cancer patientA: large esophagus mass with surface nodules (July 10, 2015); B: the uplift in the left main bronchus for external pressure (July 22, 2015)
图 1 纵隔及左肺恶性肿瘤伴食管癌待排患者胃镜及纤维支气管镜图像Figure 1 Gastroscopy and fibro bronchoscope images of mediastinal and left lung malignancy coupling with suspicion for esophagus cancer patientA: large esophagus mass with surface nodules (July 10, 2015); B: the uplift in the left main bronchus for external pressure (July 22, 2015)![]() 图 2 纵隔及左肺恶性肿瘤伴食管癌待排肿瘤组织病理图像Figure 2 Pathological images of mediastinal and left lung malignancy coupling with suspicion for esophagus cancer tissuesA: tumor cells infiltrates the submucosa layer of the esophagous wall (Hematoxylin & Eosin stain, ×100); B: positive expression of CK7 in the tumor cells (×400); C: focal positive expression of TTF-1 in the tumor cells (×400); D: negative expression of NapsinA in the tumor cells (×400); E: positive expression of ALK-Ventana IHC in the tumor cells (×100); F: ALK gene translocation demonstrated by ALK-FISH detection
图 2 纵隔及左肺恶性肿瘤伴食管癌待排肿瘤组织病理图像Figure 2 Pathological images of mediastinal and left lung malignancy coupling with suspicion for esophagus cancer tissuesA: tumor cells infiltrates the submucosa layer of the esophagous wall (Hematoxylin & Eosin stain, ×100); B: positive expression of CK7 in the tumor cells (×400); C: focal positive expression of TTF-1 in the tumor cells (×400); D: negative expression of NapsinA in the tumor cells (×400); E: positive expression of ALK-Ventana IHC in the tumor cells (×100); F: ALK gene translocation demonstrated by ALK-FISH detection![]() 图 3 纵隔及左肺恶性肿瘤伴食管癌待排患者食管造影图像Figure 3 Digital X-ray barium meal radiography of mediastinal and left lung malignancy coupling with suspicion for esophagus cancer patient' s esophagusA: esophageal lesions (arrow) before treatment (July 23, 2015); B: esophageal lesions (arrow) become smaller after treatment for 11 months (May 13, 2016)
图 3 纵隔及左肺恶性肿瘤伴食管癌待排患者食管造影图像Figure 3 Digital X-ray barium meal radiography of mediastinal and left lung malignancy coupling with suspicion for esophagus cancer patient' s esophagusA: esophageal lesions (arrow) before treatment (July 23, 2015); B: esophageal lesions (arrow) become smaller after treatment for 11 months (May 13, 2016)![]() 图 4 纵隔及左肺恶性肿瘤伴食管癌待排患者治疗期间胸部CT图像上病灶的变化Figure 4 The changes of lesions of chest CT images of mediastinal and left lung malignancy coupling with suspicion for esophagus cancer patient during the treatmentA: pulmonary lesions (arrow) before treatment (July 9, 2015); B: pulmonary lesions (arrow) become smaller after treatment for 11 months (July 4, 2016); C: enlargement of mediastinum and lung hilus lymph nodes (arrow)(July 9, 2015); D: mediastinum and lung hilus lymph nodes (arrow) become smaller after treatment for 11 months (July 4, 2016); E: mediastinal lesion (arrow) before treatment (July 9, 2015); F: mediastinal lesion (arrow) becomes smaller after treatment for 11 months (July 4, 2016)
图 4 纵隔及左肺恶性肿瘤伴食管癌待排患者治疗期间胸部CT图像上病灶的变化Figure 4 The changes of lesions of chest CT images of mediastinal and left lung malignancy coupling with suspicion for esophagus cancer patient during the treatmentA: pulmonary lesions (arrow) before treatment (July 9, 2015); B: pulmonary lesions (arrow) become smaller after treatment for 11 months (July 4, 2016); C: enlargement of mediastinum and lung hilus lymph nodes (arrow)(July 9, 2015); D: mediastinum and lung hilus lymph nodes (arrow) become smaller after treatment for 11 months (July 4, 2016); E: mediastinal lesion (arrow) before treatment (July 9, 2015); F: mediastinal lesion (arrow) becomes smaller after treatment for 11 months (July 4, 2016)2 讨论
虽然我国属于食管癌高发区,但腺癌发病率较低,且主要发生在食管下段[1]。食管腺癌常与慢性反流性食管黏膜损伤以及Barrett食管密切相关[1]。在食管癌ALK重排的报告中,国外有文献报道TPM4-ALK是唯一被识别的融合[2]。在食管腺癌和鳞癌中,ALK扩增现象很常见[3],这可能为食管癌的靶向治疗提供潜在的靶点。该患者以进行性吞咽梗阻为首发症状,同时伴进行性消瘦。胃镜查见食管中段巨大新生物,病理学提示低分化腺癌。食管造影见食管中下段狭窄、管壁僵硬、黏膜破坏,且病变范围大。胸部CT提示后纵隔软组织肿块。因此,我们高度怀疑该患者为食管癌伴远处转移。然而,患者食管病变主要在胸中段,且既往无消化道病史,故该患者食管原发腺癌的可能性小。
目前,非小细胞肺癌(NSCLC)是肺癌最常见的病理类型,占所有肺癌的80%~85%,其中,肺腺癌约占35%~40%,以周围型为主。根据报道可知,肺癌常发生脑转移,发生率约23%~65%[4]。由于绝大多数原发肺腺癌TTF-1阳性,所以它在区分肺原发腺癌与肺转移性腺癌中起重要作用。在肺腺癌中,ALK阳性概率接近2%~7%[5-6]。虽然该患者TTF-1少数细胞阳性、NapsinA阴性,不能确定为肺来源,但是从肺癌流行病学及肿瘤生物学行为方面考虑,该患者为原发性肺腺癌伴脑转移的可能性更大,后纵隔肿块考虑为纵隔转移性淋巴结肿大融合所致,食管病灶可能为肺癌转移所致,但不能完全排除合并原发性食管癌的可能。
综上,我们考虑该患者诊断为纵隔及左肺恶性肿瘤:左肺低分化腺癌伴纵隔、双侧肺门及右侧锁骨上淋巴结、脑转移,临床分期Ⅳ期,ALK阳性,食管癌不完全排除。经放疗医师评估,考虑食管穿孔风险大,暂不适宜行放疗。患者美国东部肿瘤协作组(ECOG)评分2分,ALK阳性。根据最新NCCN指南推荐:克唑替尼被批准用于ALK阳性转移性非小细胞肺癌。患者和家属要求行克唑替尼治疗。遂于2015年8月3日开始口服克唑替尼(250 mg,2次/天)治疗。患者耐受良好。服药后,自觉吞咽梗阻、咳嗽、痰中带血症状逐渐消失。至2016年7月,患者已服药11月余,未诉吞咽梗阻等不适,ECOG评分0分。复查食管造影提示食管病变缩小,长度约3.0 cm,见图 3B。胸部CT提示左下肺肿块缩小,约2.4 cm×1.9 cm;后纵隔肿块缩小,约1.7 cm×1.5 cm;纵隔及肺门淋巴结缩小,最大者短径约1.2 cm,见图 4B、4D、4F。参照实体瘤疗效评价标准(RECIST 1.1),临床疗效评价为部分缓解。患者治疗效果显著,支持我们的论断。
该患者的诊治过程告诉我们,对于病理诊断困难的特殊患者,我们需充分结合其肿瘤的生物学特征协助诊断,避免误诊、漏诊。此外,国外已有文献报道食管癌TPM4-ALK基因融合,它是否是食管癌的驱动基因,目前尚不清楚,仍需进一步探索,它可能会为食管癌的靶向治疗开辟新天地。
-
-
[1] 李婧文, 房殿春.食管腺癌相关危险因素的研究进展[J].现代消化及介入诊疗, 2014, 19(2): 94-6. http://www.cnki.com.cn/Article/CJFDTOTAL-XDXH201402009.htm Li JW, Fang DC. The research progress of esophageal adenocarcinoma associated risk factors[J].Xian Dai Xiao Hua Ji Jie Ru Zhen Liao, 2014, 19(2): 94-6. http://www.cnki.com.cn/Article/CJFDTOTAL-XDXH201402009.htm
[2] Jazii FR, Najafi Z, Malekzadeh R, et al. Identification of squamous cell carcinoma associated proteins by proteomics and loss of beta tropomyosin expression in esophageal cancer[J]. World J Gastroenterol, 2006, 12(44): 7104-12. doi: 10.3748/wjg.v12.i44.7104
[3] Schoppmann SF, Streubel B, Birner P. Amplification but not translocation of anaplastic lymphoma kinase is a frequent event in oesophageal cancer[J]. Eur J Cancer, 2013, 49(8): 1876-81. doi: 10.1016/j.ejca.2013.02.005
[4] Preusser M, Capper D, Ilhan-Mutlu A, et al. Brain metastases: pathobiology and emerging targeted therapies[J]. Acta Neuropathol, 2012, 123(2): 205-22. doi: 10.1007/s00401-011-0933-9
[5] Casaluce F, Sgambato A, Maione P, et al. ALK inhibitors: a new targeted therapy in the treatment of advanced NSCLC[J].Target Oncol, 2013, 8(1): 55-67. doi: 10.1007/s11523-012-0250-9
[6] Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK[J]. J Clin Oncol, 2009, 27(26): 4247-53. doi: 10.1200/JCO.2009.22.6993



 下载:
下载: