文章信息
- 戴勇,穆柏银,唐亚平,肖伟升,邓文兵, 廖爱军.
- DAI Yong, MU Baiyin, TANG Yaping, XIAO Weisheng, DENG Wenbing, LIAO Aijun.
- miR-711对胃癌MGC-803细胞侵袭转移及上皮间质转化的影响
- Effects of miR-711 on Invasion, Metastasis and Epithelial Mesenchymal Transition of Gastric Cancer Cells MGC-803
- 肿瘤防治研究, 2016, 43(03): 201-206
- Cancer Research on Prevention and Treatment, 2016, 43(03): 201-206
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2016.03.007
-
文章历史
- 收稿日期: 2015-05-25
- 修回日期: 2015-09-16
肿瘤侵袭转移是复杂的、多因素的、多步骤的生物学过程。近年来,上皮间质转化逐渐成为肿瘤学研究的热点。上皮间质转化(epithelial mesenchymal transition,EMT)是肿瘤发生浸润转移的早期过程,通过 EMT过程,导致细胞的黏附能力下降、迁移能力增强,使肿瘤细胞获得较高迁移、侵袭、抗凋亡和降解细胞外基质的能力,最终促进肿瘤的浸润和转移[1, 2, 3]。研究发现EMT在乳腺癌[4]、结肠癌[5]、肺癌[6]、胃癌[7]、肝癌[8]等多种癌症的原发性浸润和继发性转移中起着非常重要的作用。
微小RNA(microRNA,miRNA)是一类大小为22个核苷酸左右的单链非编码RNA,近年来研究表明,miRNAs与人类多种肿瘤的发生、发展及侵袭转移存在着密切关系[9]。miRNA-711作为 microRNAs家族成员之一,和其他miRNA分子一样与肿瘤的发生发展密切相关,为人类细胞中存在重要的调控因子。
本课题采用基因转染、划痕实验、Transwell 侵袭实验、Western blot等技术手段,研究miR-711对胃癌MGC-803细胞上皮细胞间质转化及侵袭转移的作用和机制,为早诊早治并防治胃癌转移寻找有针对性的靶标提供理论依据。
1 材料和方法 1.1 材料人胃癌 MGC-803细胞系(南华大学临床研究所赠送);质粒miRNA-711 mimics,miRNA-711 inhibitor,miRNA-NC(购买于上海吉玛制药技术有限公司);Transwell小室(8.0 μm孔径PC膜,美国Corning公司); E-cadherin、Vimentin兔抗人(美国的Cell Signaling Technology公司);β-actin兔抗人、β-actin山羊抗兔(北京博奥森公司);Matrigel胶(美国BD公司);小牛血清(浙江天杭生物科技有限公司)Lipofectamine 2000转染试剂(美国 Invitrogen公司)。
1.2 方法 1.2.1 瞬时转染在6孔板中接种对数期细胞,按5×105个/孔,在37℃、5%CO2培养箱中培养,待细胞密度为60%左右开始转染,参照Lipofectamine 2000转染试剂说明书进行转染,实验分为miR-711过表达组(miR-711 mimics组)、miR-711抑制表达组(miR-711 inhibitors组)、miRNA 空质粒组(阴性对照组)及空白转染组(空白对照组),转染后6 h换含10%小牛血清的培养液,继续培养以用于后续实验。
1.2.2 转染效率瞬时转染48 h时后,拍摄同一视野荧光下的细胞图片及非荧光下的细胞图片,随机计数100个细胞,计算含有绿色荧光细胞占细胞总数的比率,即为转染效率。
1.2.3 Transwell侵袭实验将基质胶铺于小室底部,小室下层加入含10%小牛血清的RPMI 1640培养液500 μl,小室上层加入无血清的RPMI 1640培养液100 μl,转染48 h后,用胰酶消化细胞,制成细胞悬液,小室上层铺500 μl的细胞悬液(含1×105个细胞),继续培养24 h后,4%甲醛固定、结晶紫染色后在显微镜下(100倍)随机挑取10个视野计数穿膜的细胞计算其均数和标准差,实验重复3次。
1.2.4 细胞划痕实验转染48 h后,胰酶消化制备细胞悬液接种于6孔板,待细胞长满至80%左右,用移液枪进行划痕,划“-”字形,用PBS洗2遍后,于显微镜下拍摄划痕0 h时的细胞图片,在37℃、5%CO2培养箱中培养,48 h后再于显微镜下拍摄划痕48 h的细胞图片,观察划痕处的细胞愈合情况。
1.2.5 Western blot实验转染72 h后,用胰蛋白酶消化法提取蛋白,BCA试剂盒测定蛋白含量,配平蛋白浓度,再按20 μl蛋白+5 μl蛋白上样缓冲液混匀后置于PCR仪中99℃变性10 min。配制5%浓缩胶及10%分离胶,点样后80 V电压下电泳30 min,100 V电压下电泳90 min,转膜90 min,1%封闭液封闭2 h,孵育相应一抗于4℃过夜,TBST洗3次,每次洗10 min,加二抗孵育2 h,TBST洗3次,每次洗10 min,之后放于显影仪下进行显影,用Quantity One图像分析软件对图片进行灰度值扫描,记录各组目的和内参蛋白的表达量,目的基因的蛋白相对表达量以目的/内参表示。
1.3 统计学方法所得数据采用SPSS18.0统计学软件进行分析,多个样本比较采用单因素方差分析(ANOVA),结果以均数±标准差(x±s)表示,P<0.05为差异有统计学意义。
2 结果 2.1 转染效率转染48 h后荧光显微镜下观察转染效率,转染效率=带绿色荧光细胞数目/总细胞数目,转染率约75%,见图 1。
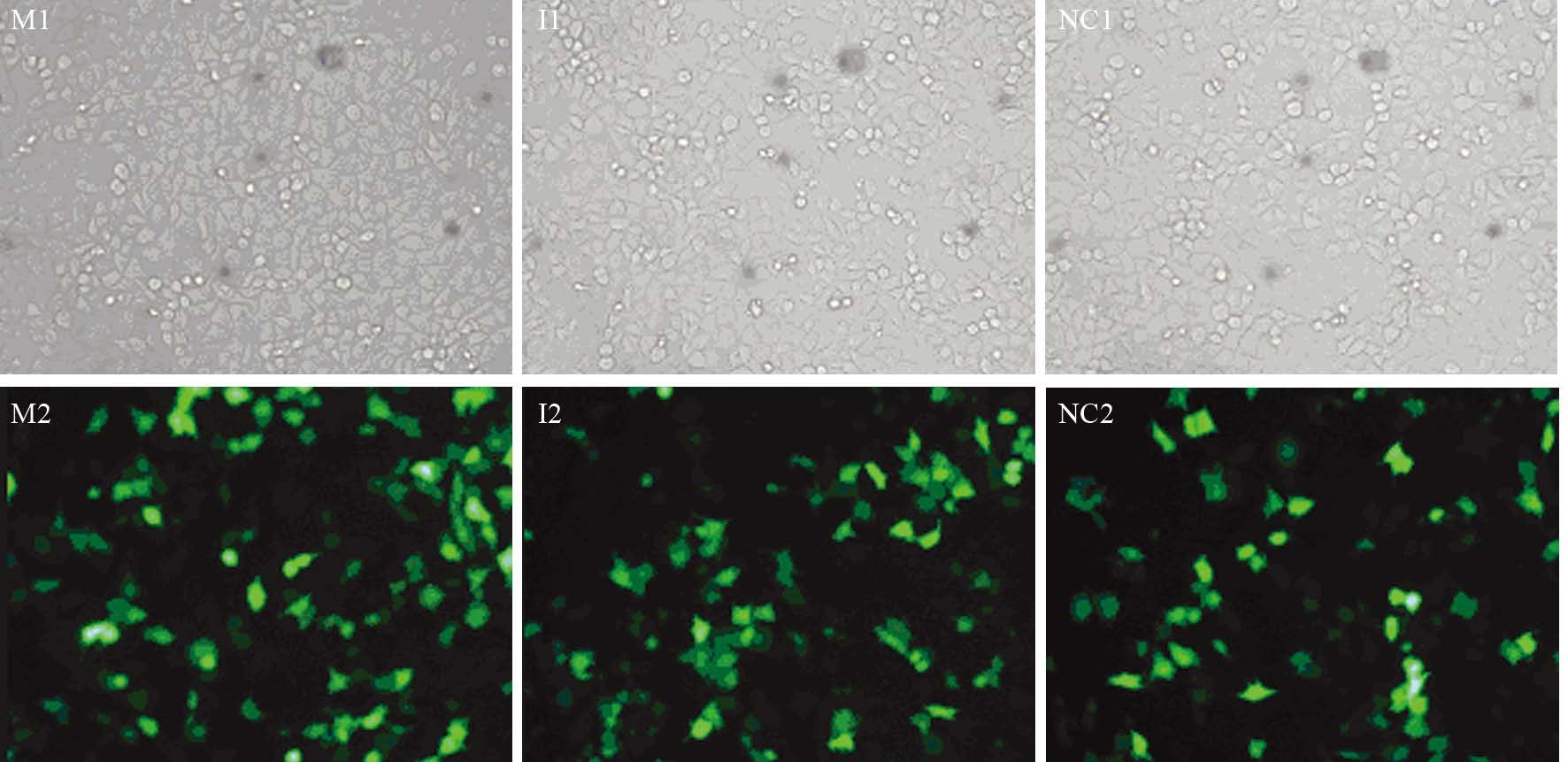
|
| M1, I1, NC1 were observed under inverted microscope; M2, I2, NC2 were observed under Fluorescence microscope; M: miR-711 mimics group; I: miR-711 inhibitors group; NC: negative control group 图 1 荧光显微镜下观察人胃癌细胞株MGC-803转染效率 (×200) Figure 1 Transfection effectiveness of human gastric cancer cell line MGC-803 observed under microscope (×200) |
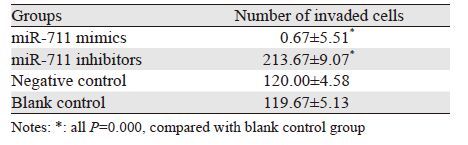
转染后48 h,Transwell侵袭实验发现:miR-711 mimics组与阴性对照组及空白对照组相比,穿膜细胞数目明显减少,差异有统计学意义(P均=0.000);miR-711 inhibitors组与阴性对照组及空白对照组相比,穿膜细胞数目明显增多,差异有统计学意义(P均=0.000);而阴性对照组与空白对照组比,差异无统计学意义(P=0.95);表明miR-711与MGC-803细胞侵袭能力负相关,miR-711可抑制MGC-803细胞侵袭,见图 2、表 1。
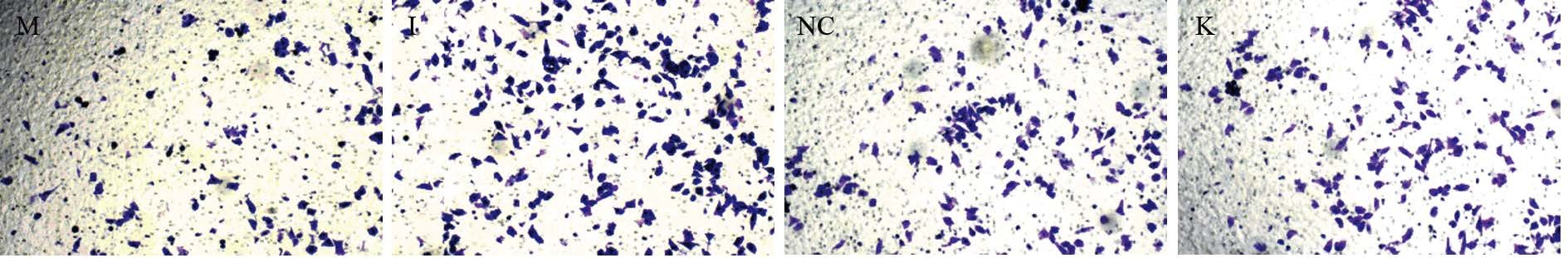
|
| K: blank control group 图 2 转染后各组胃癌MGC-803细胞穿膜数目 (×200) Figure 2 Number of invaded gastric cancer cells MGC-803 after transfection (×200) |
 |
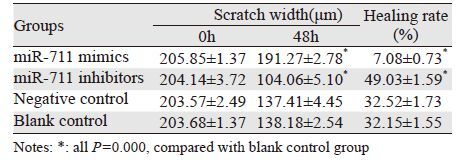
转染48 h后进行划痕,分别拍摄划痕0、48h 后细胞图片。结果显示:在划痕0 h时,各组划痕距离基本一致;划痕48 h后,miR-711 mimics组与阴性对照组及空白对照组相比,细胞愈合率明显降低,差异有统计学意义(P均=0.000);miR-711 inhibitors组与阴性对照组及空白对照组相比,细胞愈合率明显升高,差异有统计学意义(P均=0.000);而阴性对照组与空白对照组组比,差异无统计学意义(P=0.761);表明miR-711与MGC-803细胞迁移能力负相关,miR-711可抑制MGC-803细胞迁移,见图 3、表 2。
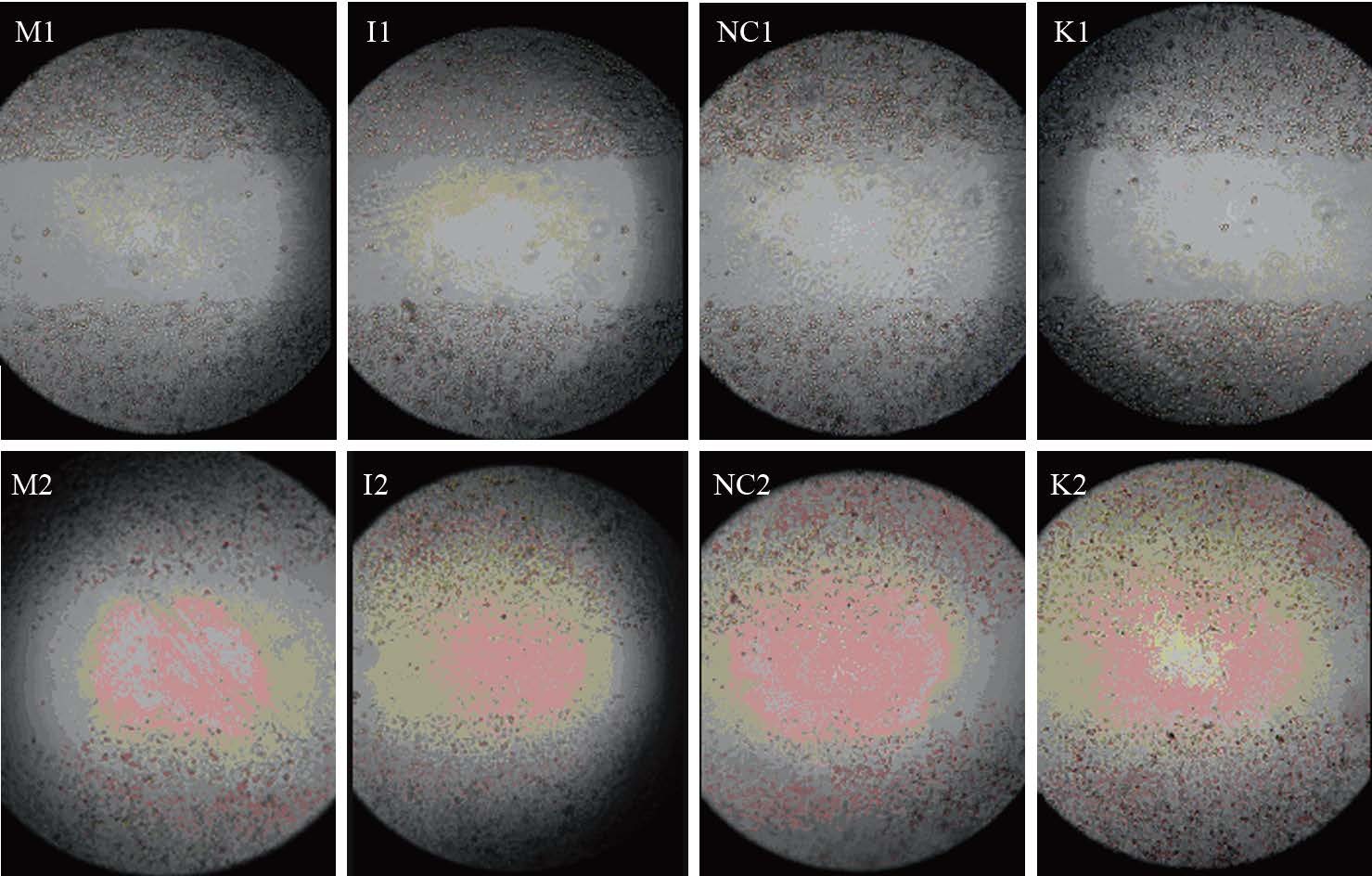
|
| M1, I1, NC1, K1: the scratch width after 0h;M2, I2, NC2, K2: the scratch width after 48h 图 3 转染后各组胃癌MGC-803细胞于0 h和48 h的划痕宽度 (×100) Figure 3 Scratch width of gastric cancer cells MGC-803 after 0 and 48h (×100) |
 |
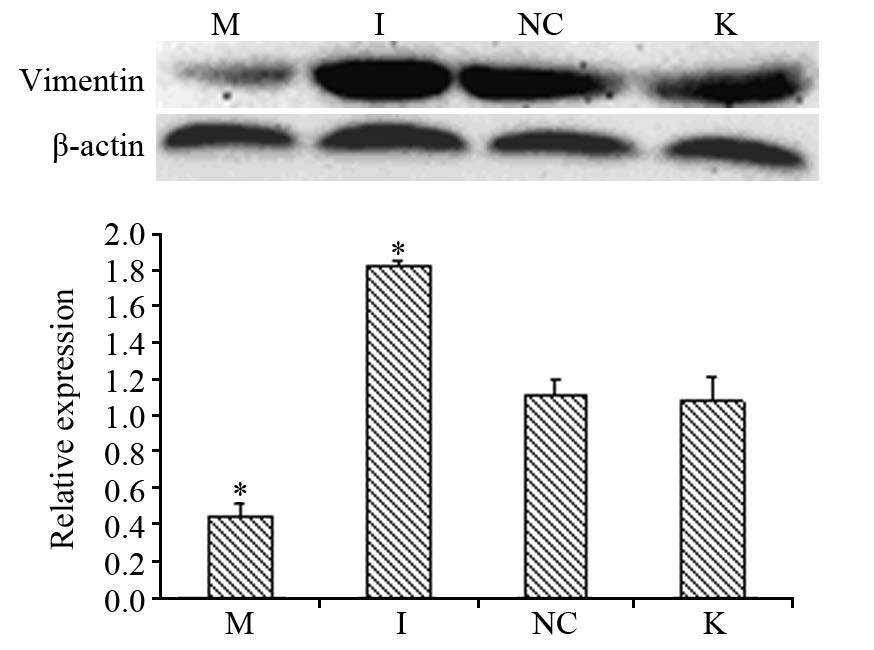
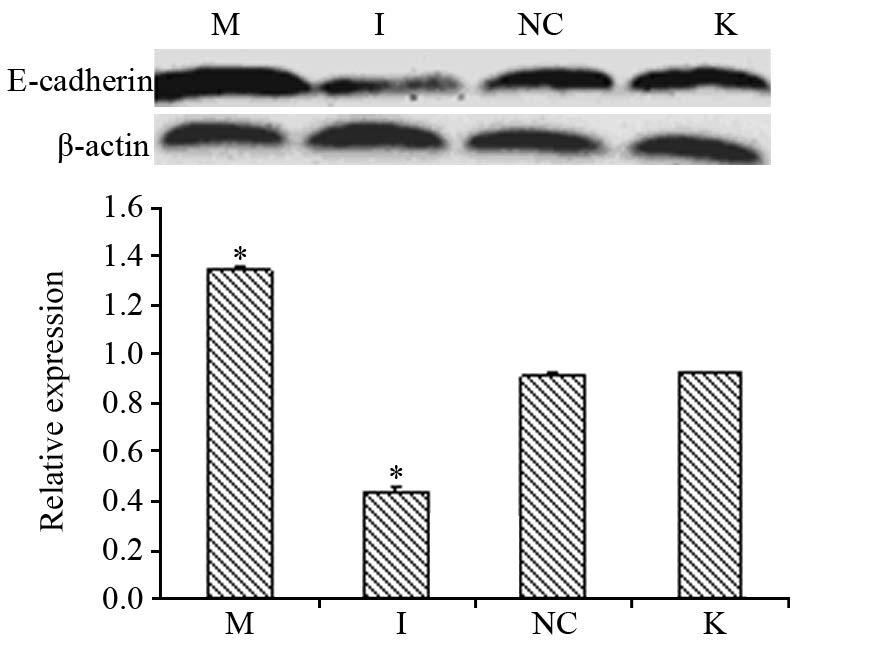
Western blot分别检测转染后各组E-cadherin蛋白、Vimentin蛋白的相对表达量,结果显示:miR-711 mimics组与阴性对照组及空白对照组相比,Vimentin蛋白相对蛋白表达量水平减少,差异有统计学意义(P均=0.000),miR-711 inhibitors组与阴性对照组及空白对照组相比,Vimentin蛋白相对蛋白表达量水平增多,差异有统计学意义(P均=0.000);而阴性对照组与空白对照组组比,差异无统计学意义(P=0.641)见图 4; miR-711 mimics组与阴性对照组及空白对照组相比,E-cadherin蛋白相对蛋白表达量增多,差异有统计学意义(P均=0.000);miR-711 inhibitors组与阴性对照组及空白对照组相比,E-cadherin蛋白相对蛋白表达量增多,差异 有 统计学意义(P均=0.000);而阴性对照组及空白对照组相比,差异无统计学意义(P=0.195)见图 5。
|
| *: all P=0.000, compared with blank control group; A: The level of Vimentin protein in gastric cancer cells MGC-803 at 72h after transfection; B: The relative expression of Vimentin protein in gastric cancer cells MGC-803 at 72h after transfection 图 4 转染72h后胃癌MGC-803细胞中Vimentin蛋白水平和 相对表达量 Figure 4 Vimentin protein and relative expression levels in gastric cancer cells MGC-803 at 72h after transfection |
|
| *: all P=0.000, compared with blank control group; A: the level of E-cadherin protein in gastric cancer cells MGC-803 at 72h after transfection; B: the relative expression of E-cadherin protein in gastric cancer cells MGC-803 at 72h after transfection 图 5 转染72 h后人胃癌MGC-803细胞中E-cadherin蛋白水 平和相对表达量 Figure 5 E-cadherin protein and relative expression levels in gastric cancer cells MGC-803 at 72h after transfection |
上皮间质化的一个重要方面是上皮标志物的缺失,尤其是E-cadherin 表达的减少,是促进肿瘤细胞运动所必需的[10, 11]。E-cadherin是一种主要的上皮标志物,其在上皮组织细胞与细胞之间的黏附及形态学的变化中起着主要作用;在原发性浸润性肿瘤中,E-cadherin升高标志着细胞与细胞之间连接的更紧密,使间质细胞向上皮细胞转变,阻碍肿瘤的浸润转移;反之则使肿瘤细胞之间相互分离,从原发部位迁移,使其具有迁移和浸润的能力,上皮细胞向间质细胞转变,促进肿瘤的浸润转移[12, 13, 14]。大量研究揭示,在肿瘤的发生过程中,存在上皮标志物E-cadherin的表达下调及间质标志物Vimentin的表达上调,说明肿瘤的发生涉及EMT[15, 16, 17]。研究发现:有许多miRNAs参与EMT调节[18, 19],目前发现miR-200家族成员miR-200a、miR-200b、miR-200c、miR-141和miR-429是EMT的重要调节因子,通过上调上皮标志物E-cadherin、下调间质标志物Vimentin的表达抑制肿瘤EMT过程 [20, 21, 22] ,同时有研究发现miR-200家族可靶向作用于ZEB1/ZEB2 ,间接或直接抑制上皮间质转化的过程[23, 24, 25]。为了进一步研究miR-711是否参与胃癌 MGC-803细胞上皮间质转化过程,本研究选择上皮标志物 E-cadherin、间质标志物Vimentin 作为判断指标,利用Western blot实验检测胃癌MGC-803细胞上皮标志物E-cadherin、间质标志物Vimentin的蛋白表达情况,探讨miR-711对胃癌MGC-803 细胞 EMT 的影响;利用Transwell侵袭小室实验检测细胞的侵袭能力,细胞划痕实验检测细胞的迁移能力;探讨miR-711对胃癌MGC-803 细胞侵袭转移的影响
Transwell侵袭小室实验表明miR-711与胃癌MGC-803细胞侵袭能力负相关;划痕实验显示miR-711与MGC-803细胞迁移能力负相关;Western blot结果说明miR-711可维持上皮极性或是促使肿瘤细胞由间质表型向上皮表型转化。综上表明:miR-711能抑制胃癌 MGC-803细胞的侵袭转移和上皮间质转化,这为进一步研究miR-711的抑癌机制,为胃癌的防治提供了理论依据。
CD44是一种细胞黏附分子,被认为是胃癌干细胞的表面标志物,与肿瘤的分化、转移、体外成瘤及耐药相关[26]。有研究发现:TGF-β1处理胃癌SGC7901细胞后CD44表达明显增加,EMT相关因子表达同时增高[27],说明CD44与EMT存在相关性。生物信息学分析发现CD44为miR-711的靶基因之一。关于miR-711是否通过调控CD44的表达来影响胃癌细胞发生EMT,将是本课题下一步将要研究的内容。
| [1] | Verdecchia A, Corazziari I, Gatta G, et al. Explaining gastric cancer survival differences among European countries[J]. Int J Cancer, 2004, 109(5): 737-41. |
| [2] | Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease[J]. Cell, 2009, 139(5): 871-90. |
| [3] | Rosivatz E, Becker I, Specht K, et al. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer[J]. Am J Pathol, 2002, 161(5): 1881-91. |
| [4] | Yan W, Cao QJ, Arenas RB, et al. GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition[J]. J Biol CHem, 2010, 285(18): 14042-51. |
| [5] | Wilmanns C, Steinhauer S, Grossmann J, et al. Site-dependent differences in clinical,pathohistolo-gical,and molecular parameters in metastastatic colon cancer[J]. Int J Biol Sci, 2009, 5(5): 458-65. |
| [6] | Davis R, Rizwani W, Banerjee S, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer[J]. PLoS One, 2009, 4(10): e7524. |
| [7] | Kurashige J, Kamohara H, Watanabe M, et al. MicroRNA-200b reguletes cell proliferation,invision, and migration by directly targeting ZEB2 in gastric carcinoma[J]. Ann Surg Oncol, 2012, Suppl 3: S656-64. |
| [8] | Meng Z, Fu X, Chen X, et al. miR-194 is a maker of hepatic epithelial cell and suppresses metastasis of liver cancer cells in mice[J]. Hepatology, 2010, 52(6): 2148-57. |
| [9] | Zhou W, Li X, Liu F, et al. MiR-135a promotes growth and invasion in colorectal cancer via metastasis suppressor 1 in vitro[J]. Acta Biochim Biophys Sin(Shanghai), 2012, 44(10): 838-46. |
| [10] | Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy[J]. Clin Cancer Res, 2009, 15(16): 5060-72. |
| [11] | Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2[J]. Oncogene, 2007, 26(5): 711-24. |
| [12] | Thiery JP. Epithelial-mesenchymal transitions in tumour progression[J]. Nat Rev Cancer, 2002, 2(6): 442-54. |
| [13] | Du C, Zhang C, Hassan S, et al. Protein kinase D1 suppresses epithelial-to-mesenchymal transition through phosphorylation of Snail[J]. Cancer Res, 2010, 70(20): 7810-9. |
| [14] | Shioiri M, Shida T, Koda K, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal cancer patients[J]. Br J Cancer, 2006, 94(12): 1816-22. |
| [15] | Castro Alves C, Rosivatz E, Schott C, et al. Slug is overexpressed in gastric cancers and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin[J]. J Pathol, 2007, 211(5): 507-15. |
| [16] | Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis[J]. Dev Cell, 2008, 14(6): 818-29. |
| [17] | Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease[J]. J Cell Biol, 2006, 172(7): 973-81. |
| [18] | Smith AL, Iwanaga R, Drasin DJ, et al. The miR-106b-25 cluster targets Smad7, activates TGF-β signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer[J]. Oncogene, 2012, 31(50): 5162-71. |
| [19] | Chiou GY, Cherng JY, Hsu HS, et al. Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial-mesenchymal transdifferentiation and cancer stem-like properties and in lung Aden carcinoma[J]. J Control Release, 2012, 159(2): 240-50. |
| [20] | Turcatel G, Rubin N, El-Hashash A, et al. mir-99a and MIR-99b modulate TGF-β induced epithelial to mesenchymal plasticity in normal murine mammary gland cells[J]. PLoS One, 2012, 7(1):e31032. |
| [21] | Schliekelman MJ, Gibbons DL, Faca VM, et al.Targets of the tumor suppressor miR-200 in regulation of the epithelial-mesenchymal transition in cancer[J]. Cancer Res, 2011, 71(24): 7670-82. |
| [22] | Tellez CS, Juri DE, Do K, et al. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells[J]. Cancer Res, 2011, 71(8): 3087-97. |
| [23] | Paterson EL, Kazenwadel J, Bert AG, et al. Down-regulation of the miRNA-200 family at the invasive front of colorectal cancers with degraded basement membrane indicates EMT is involved in cancer progression[J]. Neoplasia, 2013, 15(2): 180-91. |
| [24] | Gregory PA, Bracken CP, Smith E, et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition[J]. Mol Biol Cell, 2011, 22(10): 1686-98. |
| [25] | Ahmad A, Aboukameel A, Kong D, et al. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells[J]. Cancer Res, 2011, 71(9): 3400-9. |
| [26] | Jang BI, Li Y, Graham DY, et al. The role of CD44 in the pathogenesis, diagnosis, and therapy of gastric cancer[J]. Gut Liver, 2011, 5(4): 397-405. |
| [27] | Cai C, Yu JW, Wu JG, et al. Transforming growth factor-β1 generates epithelial-to-mesenchymal transition and promote CD44 expression in SGC7901 cells[J]. Guo Ji Wai Ke Xue Za Zhi, 2012, 39(11): 746-51, cover3-4. [蔡成, 俞继卫, 吴巨钢, 等. TGF-β1诱导胃癌细胞上皮间质转化及其上调CD44表达的实验研究[J]. 国际外科学杂志 , 2012, 39(11): 746-51, 封3-4.] |
 2016, Vol. 43
2016, Vol. 43


