文章信息
- 卢景宁,杨红,彭民浩,肖开银,彭涛,彭金波. 2015.
- LU Jingning, YANG Hong, PENG Minhao, XIAO Kaiyin, PENG Tao, PENG Jinbo. 2015.
- 人工腹水辅助超声引导射频消融治疗膈顶部原发性肝癌
- Percutaneous Ultrasound-guided Radiofrequency Ablation with Artificial Ascites for Hepatocellular Carcinoma in Hepatic Dome
- 肿瘤防治研究, 2015, 42(05): 493-497
- Cancer Research on Prevention and Treatment, 2015, 42 (05): 493-497
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2015.05.016
-
文章历史
- 收稿日期:2014-11-20
- 修回日期:2014-12-19
2. 530021 南宁,广西医科大学第一附属医院超声诊断科
2. Department of Ultrasound, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, China
射频消融(radiofrequency ablation,RFA)是肝细胞癌(hepatocellular carcinoma,HCC)的根治性治疗方法之一,对小肝癌的治疗效果可达根治性切除术水平[1, 2, 3],RFA对于合并肝硬化或术后复发无法行切除术的HCC患者具有重要意义。目前临床RFA治疗通常在超声引导下进行,但由于超声技术自身的局限性,使得部分困难部位(如膈顶部位置)的病灶在肺气干扰下难以经超声引导行RFA治疗,为此本研究尝试建立人工腹水辅助超声引导RFA治疗膈顶部HCC,现报道如下。 1 资料与方法 1.1 临床资料
选择2010年1月至2012年12月间在广西医科大学第一附属医院肝胆外科因膈顶部HCC自愿行人工腹水辅助超声引导下经皮RFA治疗的22例患者的25个病灶,男18例、女4例,平均年龄(54.1±10.6)岁;Child-Pugh分级为A级17例、B级5例。19例患者为单发病灶、3例患者为多发病灶;肿瘤最大直径4.0 cm,最小直径1.8 cm,平均(2.7±0.6) cm;有3个病灶位于肝Ⅳ段、13个病灶位于肝Ⅶ段、9个位于肝Ⅷ段;15个病灶因常规B型超声不能完全显示病灶范围或位置、10个病灶因常规超声引导下穿刺径路被肺气或肋骨阻挡无合适的穿刺路径需要行人工腹水辅助。
纳入研究标准[4, 5]:(1)肿瘤位于膈顶部或毗邻膈肌,常规B型超声不能完全显示病灶范围或位置。(2)常规超声引导下穿刺径路被肺气或肋骨阻挡无合适的穿刺路径进。(3)单发肿瘤,最大直径≤5 cm;或肿瘤数目≤3个,且最大直径≤3 cm。(4)肿瘤无血管、胆管和邻近器官侵犯以及远处转移。(5)患者肝功能分级为Child Pugh A或B级,或经内科护肝治疗达到该标准。 1.2 人工腹水的操作方法
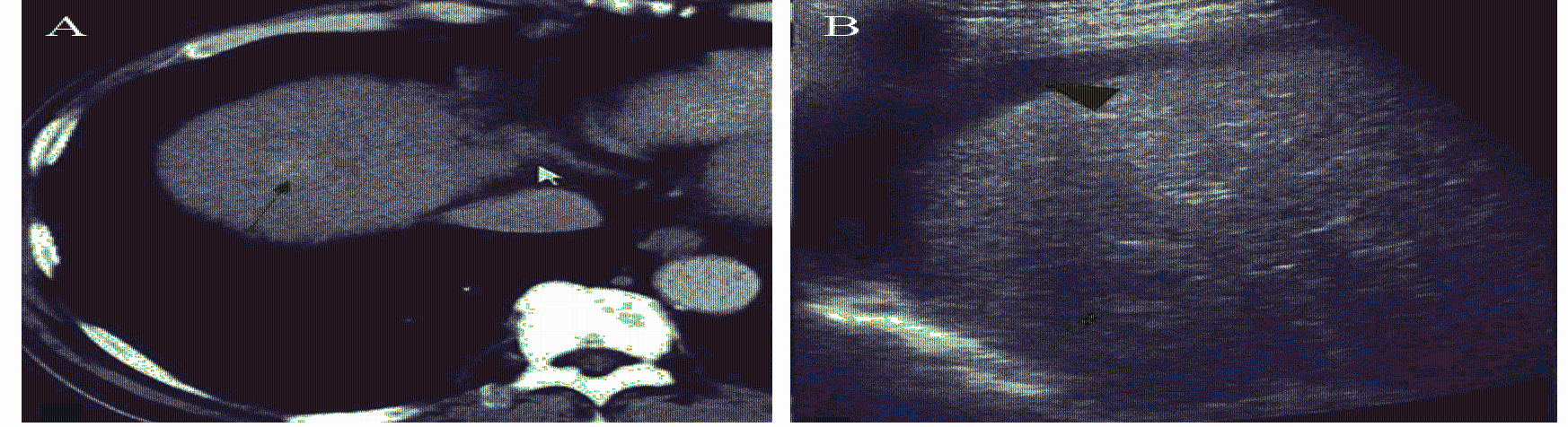
人工腹水建立的方法参照Seldinger穿刺技术[6, 7, 8]并进行改进:患者取仰卧位,常规消毒铺巾,取第7~8肋间隙与右腋前线交点为穿刺点,2%利多卡因局麻穿刺点至腹膜,超声实时引导下,用5-F动脉鞘穿刺套件(Terumo corporation,Japan)21G穿刺针穿刺入肝与腹膜之间的间隙后退出针芯,在超声实时引导下沿套管将导丝置入右侧膈顶部,将5-F动脉鞘管沿导丝方向置入右侧膈顶部,经动脉鞘滴注5%葡萄糖溶液750~1 500 ml[9],见图 1。实时超声监测腹腔内人工腹水厚度,直到肝脏上界下移,肝脏与膈肌分离,膈顶部肿瘤位置显示清楚,穿刺路径完全显露,视为人工腹水建立成功,见图 2。
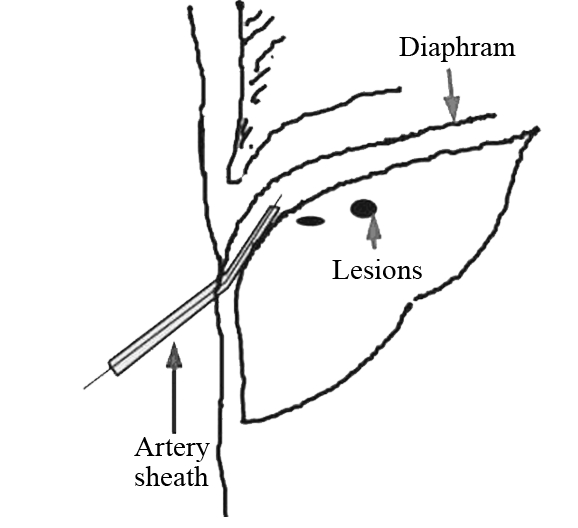
|
| 图1 建立人工腹水的方法 Figure 1 Establishment methods of artificial ascites |

|
| A: ultrasound was unable to display some lesions in the hepatic dome before artificial ascites; B: after the establishment of artificial ascites, the lesions could be fully displayed by ultrasound. C: after the establishment of artificial ascites, hepatocellular carcinoma in the hepatic dome could be fully displayed by ultrasound 图2 建立人工腹水效果图 Figure 2 Schematic diagram of establishment process of artificial ascites |
患者成功建立人工腹水后,见图 3,应用RF-2000TM射频治疗仪及集束电极针(Radio Therapeutics Corp,USA),在LOGIQ E9超声诊断仪(General Electric Company,USA)实时引导下将射频针穿刺入病灶底部,采用多点逐层布针方式,每层5 mm,逐层打开治疗伞进行RFA治疗,要求消融范围覆盖整个肿瘤,并且超出肿瘤边缘5~10 mm。射频治疗仪输出功率由30 W逐步增加至80 W直至RFA治疗完全,以防止针道转移,最后拔针前注意针道烧灼,穿刺点止血。
|
| A: The lesions were located in the diaphragm dome(fine red arrow) before radiofrequency ablation(RFA) showed by CT; B: After the establishment of artificial ascites(thick red arrow), the lesions in the back diaphragm dome could be fully displayed by ultrasound(fine red arrow);C: Percutaneous ultrasound-guided RFA with artificial ascites (thick red arrow) for hepatocellular carcinoma in the hepatic dome(fine red arrow);D: After RFA, tumor (thin red arrow) was completely ablated, and concurrent reactive pleural effusion (thick blue arrows) was showed by CT 图3 人工腹水辅助射频消融治疗膈顶部原发性肝癌前、治疗中及治疗后示意图 Figure 3 Schematic diagram of hepatocellular carcinoma in hepatic dome before, during and after artificial ascites with RFA treatment |
患者行RFA治疗后第1、3、7天进行常规B型超声检查,观察人工腹水量,记录有无出现胸腔积液、膈肌损伤、血胸、肝被膜下出血、腹腔出血及肝脓肿等术后并发症。RFA治疗后1月后行增强CT或者增强MR检查评估肿瘤是否消融完全,有无残留,见图 3。肿瘤残留的判断标准[10, 11]:肿瘤RFA治疗后原射频位置肿瘤体积明显增大并存在内部或者边缘周围有增强的局部复发,残留病灶表现为增强CT/MRI检查出现动脉期增强、门脉期消退的典型影像学表现。RFA治疗后随访2~3月观察肿瘤有无进展。如治疗后1月增强CT或增强MR未见肿瘤增强,同时随访2~3月肿瘤无进展则认为肿瘤消融完全。 1.5 统计学方法
使用SPSS17.0统计软件对数据进行收集、整理及分析。计量资料结果以(x±s)表示。 2 结果 2.1 人工腹水的可行性及有效性
所有22例患者参照Seldinger穿刺方法均置管成功,5%葡萄糖溶液的注入量为(943.2±187.9) ml。但仅19例(19/22,86.4%)患者22个病灶(22/25,88.0%)成功在膈下形成局限性的人工腹水,厚度(肝右叶顶部距膈肌的最大距离)为(3.4±1.3)mm。22个成功建立人工腹水的病灶中,13个由于肺气的遮挡常规超声不能显示完全病灶范围,9个穿刺路径被肺气或肋骨阻挡,建立人工腹水后所有病灶位置及范围完全显示,并清晰显示穿刺路径。3例患者3个病灶(3/25,12.0%)注入5%葡萄糖溶液后未能在局部形成局限性的人工腹水,其中2例患者为HCC手术切除后复发病例,腹腔粘连严重,注入5%葡萄糖溶液后仍无法将肝脏与膈肌分离,1例为肿瘤位于肝Ⅷ段肝裸区内,注入5%葡萄糖溶液亦无法令肝脏与膈肌分离,故3例患者在无人工腹水辅助下行RFA治疗,术后一个月增强CT/MR随访复查病灶消融完全,但1例患者局部膈肌血供消失。其余19例患者22个病灶在人工腹水辅助下完成RFA治疗,治疗后一个月增强CT/MR随访复查均提示消融完全,膈肌血供正常。 2.2 人工腹水的安全性
本研究组未发生腹腔出血、腹膜炎、血胸、气胸、膈肌损伤、胆瘘、肠瘘、肝脓肿及肿瘤针道转移等严重并发症,无手术相关死亡病例。术后监测人工腹水量,所有病例均于治疗后3天内人工腹水完全吸收。3例患者出现少量胸腔积液,未进行特殊处理,于术后7天内完全自行吸收。8例患者RFA治疗后出现右侧肩胛区疼痛,予常规对症治疗后好转。 3 讨论
临床上RFA治疗凭借其有效性、微创性、可重复性及安全性等优势,已不仅仅局限于无法手术切除的HCC,还广泛地应用于治疗小肝癌或等待肝移植前的一线治疗之中[12, 13]。由于超声无法穿透气体与骨骼的局限性,肝脏膈顶部被称为超声“盲区”。当位于该区域的病灶行RFA治疗时,由于受到肺气或肋骨的干扰,超声引导穿刺往往会出现不能完全显示肿瘤、穿刺径路被肺气或肋骨阻挡无合适的穿刺路径等问题影响治疗的进行和效果,如勉强进行超声引导下RFA治疗则可能会出现肿瘤消融不完全或是损伤膈肌、心肺等重要邻近脏器的问题。有文献报道[14, 15, 16, 17]建立人工胸水可解决该问题。胸腔内注入的液体可使肺气上移,肺气与膈分离,透声性良好的液体使超声可清晰的显示膈顶部的肝肿瘤病灶。但人工胸水可能导致胸腔积液、气胸、血胸、脓胸、呼吸衰竭等并发症[18]。并且人工胸水涉及到跨学科操作的问题,临床实践中往往会遇上一定的困难。
本研究通过在超声实时引导下于腹腔右侧膈顶部置入导管后注入5%葡萄糖溶液建立人工腹水,可使肝脏上界下移避免肺气干扰从而显示近膈顶部的病灶。同时能避免液体进入胸腔引起的相应肺部并发症,该操作简单易行[19]。本研究结果显示:在建立人工腹水后,膈顶部病灶得以充分暴露,穿刺径路清晰,同时RFA区域与膈肌之间形成一道人工腹水保护层。根据国外一些研究资料[7, 20],为避免邻近膈肌出现热损伤,人工腹水厚度至少应大于2 mm,为保护邻近重要脏器人工腹水厚度至少大于5 mm,本研究组人工腹水厚度为(3.4±1.3)mm。本组病例未发生腹腔出血、腹膜炎、血胸、气胸、膈肌损伤、胆瘘、肠瘘、肝脓肿及肿瘤针道转移等并发症,无手术相关死亡病例。术后1月增强CT/MR检查及随访显示所有病灶均消融完全。
由于平卧位膈下并非腹腔最低位置,因此注入的液体并非首先积聚于膈下部位。为提高人工腹水的成功率,避免在腹腔注入过多的液体,本研究还改进了人工腹水建立方法:采用5-F动脉鞘穿刺套件参照Seldinger穿刺技术进行置管,将5-F动脉鞘管沿导丝置入右侧膈顶部接近病灶的肝膈间隙后注入5%葡萄糖溶液,使人工腹水首先积聚于右膈顶近病灶处,提高了人工腹水的成功率。本研究中,注入的人工腹水量为(943.2±187.9)ml,形成的人工腹水厚度为(3.4±1.3)mm。同时,治疗过程中超声实时动态监测人工腹水情况,保持动脉鞘管的通畅,随时调节人工腹水的量。治疗结束后,开放动脉鞘管,引流出注入的人工腹水后拔除鞘管,本组所有病例均能将大部分腹水引出,术后超声观察腹水情况显示所有腹水均在3天内完全吸收。因此认为应用5-F动脉鞘管留置法建立人工腹水,操作简单、成本低廉、成功率高、易于复制及推广。与国内外一些中心所采用的人造胸水辅助RFA治疗相比较[14, 15, 17],无需安置胸腔引流装置,患者减少了胸腹腔置管的痛苦,同时避免跨学科操作,减少胸腔积液,血气胸,肺部感染,损伤心肺等并发症的发生。但是,本组病例亦提示了人工腹水辅助超声引导RFA治疗膈顶部HCC仍存在不足之处。分析本研究组中3例未能成功进行人工腹水辅助RFA治疗的病例,其中2例为HCC术后复发,腹腔粘连严重,注入人工腹水后无法将RFA区域与膈肌分离,1例肿瘤位于肝裸区,建立人工腹水后,虽然肝上缘下移但肿瘤位置仍无法完全显示。提示人工腹水条件下针对这两类病例的治疗存在困难,且这3例病例肝功能较差或合并其他器官疾病会导致手术耐受性差,最终采用了人工胸水辅助下行RFA治疗。
综上所述,原位于超声盲区无法进行RFA治疗的膈顶部肿瘤在建立人工腹水后能清楚显示,使部分困难病例获得了治疗机会,并保证治疗的完全性及安全性,拓宽了RFA治疗适应证;人工腹水辅助超声引导RFA治疗膈顶部HCC安全、简易、可行,有较高临床应用价值。
| [1] | Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010[J]. Oncology, 2010, 78 Suppl 1: 113-24. |
| [2] | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update[J]. Hepatology, 2011, 53(3): 1020-2. |
| [3] | Luo DY, Xu GL, Jia WD, et al. Surgical treatment of hepatocellular carcinoma with portal hypertension[J]. Zhong Liu Fang Zhi Yan Jiu, 2014, 41(8): 925-7. [罗大勇, 许戈良, 荚卫东, 等. 肝细胞性肝癌合并静脉高压症的外科治疗[J]. 肿瘤防治研究, 2014, 41(8): 925-7.] |
| [4] | Kang TW, Rhim H, Lee MW, et al. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy[J]. AJR Am J Roentgenol, 2011, 196(4): 907-13. |
| [5] | Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors[J]. J Hepatol, 2013, 58(1): 89-97. |
| [6] | O’Neill MJ, Weissleder R, Gervais DA, et al. Tunneled peritoneal catheter placement under sonographic and fluoroscopicguidance in the palliative treatment of malignant ascites[J]. AJR Am J Roentgenol, 2001, 177(3): 615-8. |
| [7] | Rhim H, Lim HK, Kim YS, et al. Percutaneous radiofrequency ablation with artificial ascites for hepatocellularcarcinoma in the hepatic dome: initial experience[J]. AJR Am J Roentgenol, 2008, 190(1): 91-8. |
| [8] | Akinci D, Erol B, Ciftci TT, et al. Radiologically placed tunneled peritoneal catheter in palliation of malignantascites[J]. Eur J Radiol, 2011, 80(2): 265-8. |
| [9] | Laeseke PF, Sampson LA, Brace CL, et al. Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water[J]. AJR Am J Roentgenol, 2006, 186(5 Suppl):S249-54. |
| [10] | Kei SK, Rhim H, Choi D, et al. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence[J]. AJR Am J Roentgenol, 2008, 190(6): 1544-51. |
| [11] | Chopra S, Dodd GD 3rd, Chintapalli KN, et al. Tumor recurrence after radiofrequency thermal ablation of hepatic tumors: spectrum of findings on dual-phase contrast-enhanced CT[J]. AJR Am J Roentgenol, 2001, 177(2): 381-7. |
| [12] | DuBay DA, Sandroussi C, Kachura JR, et al. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation[J]. HPB (Oxford), 2011, 13(1): 24-32. |
| [13] | Schroeder T, Sotiropoulos GC, Molmenti EP, et al. Changes in staging for hepatocellular carcinoma after radiofrequency ablation prior to liver transplantation as found in the explanted liver[J]. Hepatogastroenterology, 2011, 58(112): 2029-31. |
| [13] | Fukuno H, Tamaki K, Urata M, et al. Influence of an artificial pleural effusion technique on cardio-pulmonary function and autonomic activity[J]. J Med Invest, 2007, 54(1-2): 48-53. |
| [15] | Iwai S, Sakaguchi H, Fujii H, et al. Benefits of artificially induced pleural effusion and/or ascites for percutaneous radiofrequency ablation of hepatocellular carcinoma located on the liver surface and in the hepatic dome[J]. Hepatogastroenterology, 2012, 59(114): 546-50. |
| [16] | Liu LN, Xu HX, Lv MD. Percutaneous ultrasound-guided ablation for liver tumor with artificial pleural effusion[J]. Zhonghua Yi Xue Chao Sheng Za Zhi (Dian Zi Ban), 2012, 9(12): 1034-9. [刘琳娜, 徐辉雄, 吕明德. 人工胸腔积液在超声引导经皮消融肝肿瘤中的应用[J]. 中华医学超声杂志(电子版), 2012, 9(12): 1034-9.] |
| [17] | Zhang D, Liang P, Yu X, et al. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study[J]. Int J Hyperthermia, 2013, 29(7): 663-70. |
| [18] | Koda M, Ueki M, Maeda Y, et al. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm[J]. AJR Am J Roentgenol, 2004, 183(3): 583-8. |
| [19] | Nishimura M, Nouso K, Kariyama K, et al. Safety and efficacy of radiofrequency ablation with artificial ascites for hepatocellular carcinoma[J]. Acta Med Okayama, 2012, 66(3): 279-84. |
| [20] | Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites[J]. Abdom Imaging, 2009, 34(3): 371-80. |
 2015, Vol. 42
2015, Vol. 42


