文章信息
- 孙启天,高宇,刘晓燕,贺晓磊,邢恩鸿,孙立新. 2015
- SUN Qitian, GAO Yu, LIU Xiaoyan, HE Xiaolei, XING Enhong, SUN Lixin. 2015
- 高糖、高胰岛素对乳腺癌MCF-7细胞腺苷酸活化蛋白激酶表达的影响及二甲双胍的干预研究
- Effects of High Glucose and High Insulin on Adenosine Monophosphate-activated Protein Kinase Expression in Breast Cancer Cells MCF-7 and Metformin Intervention
- 肿瘤防治研究, 2015, 42(04): 328-333
- Cancer Research on Prevention and Treatment, 2015, 42(04): 328-333
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2015.04.003
-
文章历史
- 收稿日期:2014-03-13
- 修回日期:2014-08-07
2. 067000 承德,承德医学院附属医院中心实验室
2. Department of Central Laboratory, The Affiliated Hospital of Chengde Medical College, Chengde 067000, China
肿瘤与2型糖尿病具有一些共同的危险因素,例如肥胖、饮酒、吸烟等。人们陆续发现2型糖尿病患者的多种肿瘤发病率及死亡率有所升高且降糖药二甲双胍可能具有一定抗肿瘤作用[1, 2, 3],具体机制不明,现普遍认为与二甲双胍的作用靶点腺苷酸活化蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK)的激活有关。AMPK是一种重要的细胞能量调节因子,磷酸化的腺苷酸活化蛋白激酶(p-AMPK)可以使血糖、血脂下降,缓解疾病症状[4]。本研究选用AMPKα1作为检测对象,通过反映不同条件下AMPK的变化,探讨AMPK在乳腺癌及糖尿病关系中的作用,及二甲双胍对糖尿病合并乳腺癌患者肿瘤的抑制机制。1 材料与方法1.1 材料
人乳腺癌MCF- 7细胞株由承德医学院附属医院中心实验室提供,盐酸二甲双胍、D-葡萄糖(D-glucose)购自上海生工生物公司,低糖DMEM培养液购自美国Hyclone公司,胎牛血清购自浙江天杭生物科技有限公司,胰蛋白酶购自美国Invitrogen公司,胰岛素购丹麦自诺和诺德公司,BCA蛋白定量试剂盒购自联科生物科技有限公司,兔抗人AMPKα1、p-AMPKα1/2(pT183/pT172)单克隆抗体购自英国abcam公司,兔抗人β-actin单克隆抗体购自杭州华安生物技术有限公司,羊抗兔IgG二抗购自英国abcam公司,引物合成购自美国Invitrogen上海贸易有限公司,TRIzol reagent购自美国Invitrogen公司,M-MLV第一链合成试剂盒购自上海英骏生物技术有限公司,2×Taq Master Mix购自北京康为试剂生物有限公司,细胞周期与细胞凋亡检测试剂盒购自江苏碧云天生物技术研究所。1.2 实验方法1.2.1 细胞培养
用含1 0 %胎牛血清的低糖DMEM培养液在37℃、5%CO2的细胞培养箱中培养MCF-7细胞。1.2.2 实验分组
细胞分为:高糖组、高胰岛素组、高糖+高胰岛素组,其中高糖组含葡萄糖浓度20 mmol/L、胰岛素浓度0 mu/L;高胰岛素组含葡萄糖浓度5 mmol/L、胰岛素浓度125 mu/L;高糖+高胰岛素组含葡萄糖浓度20 mmol/L、胰岛素浓度125 mu/L。此外,分别用浓度为0、2.5、5、10和20 mmol/L的二甲双胍对各组细胞进行干预,以二甲双胍浓度为0 mmol/L时作为对照组。1.2.3 Western blot方法检测AMPKα1及p-AMPKα1/2的蛋白表达
取对数生长期细胞,接种于25 ml细胞培养瓶中,过夜,使细胞贴壁,更换不同培养液,继续培养72 h。提取蛋白,进行BCA蛋白定量,煮沸蛋白,配胶,加样后进行电泳,转模,封闭。将膜置于AMPKα1、p-AMPKα1/2、β-actin一抗中4℃孵育过夜,洗膜后将膜置于二抗中孵育1 h。曝光,定影。扫描胶片并用软件进行分析。1.2.4 RT-PCR检测AMPKα1 mRNA的表达
取对数生长期细胞,过夜,使细胞贴壁后,更换不同培养液,继续培养72 h,提取MCF-7细胞RNA,检测RNA浓度及纯度,于-80℃保存,取1 μg反转录为cDNA,以cDNA为模板进行扩增,其中AMPKα1 mRNA引物序列为正义序列:5’-GGCACGCCATACCCTTGA-3’,反义序列:5’-TCTTCCTTCGTACACGCAAATAA-3’。RT-PCR产物长度为194 bp,扩增条件为预变性95℃ 5 min,变性95℃ 45 s,退火60℃ 45 s,延伸72℃,1 min,共38个循环,最后72℃延伸8 min。取5 μl扩增产物进行2%琼脂糖凝胶电泳,并用软件进行分析。1.2.5 流式细胞仪检测细胞周期
取对数生长期MCF-7细胞,调整细胞浓度,更换培养液,继续培养72 h,按照试剂盒说明书固定、洗涤,PI染色,37℃避光放置30 min,置于流式细胞仪488 nm波长处检测。1.3 统计学方法
采用SPSS17.0软件进行统计学分析。数据用x±s表示 ,两组数据间比较采用独立样本t检验,多组数据间比较采用单因素方差分析,P<0.05为差异有统计学意义。2 结果 2.1 高糖+高胰岛素干预下AMPKα1及p-AMPKα1/2蛋白的表达水平
在高糖+高胰岛素作用条件下,p-AMPKα1/2及 AMPKα1/2 的蛋白表达较对照有所下降,差异均有统计学意义(P=0.02、0.01),见图 1。
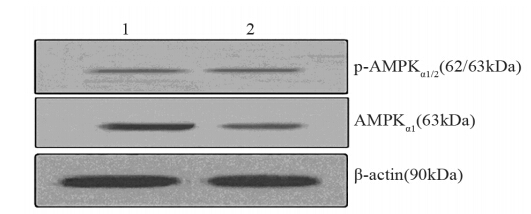 |
| 1: control group; 2: 20mmol/L glucose+125mu/L insulin group 图 1 Western blot检测高糖+高胰岛素作用下AMPKα1及p-AMPKα1/2蛋白表达水平 Figure 1 The expression of AMPKα1 and p-AMPKα1/2 protein in MCF-7 cells in high glucose plus high insulin group detected by Western blot |
用不同浓度二甲双胍(0、2.5、5、10和20 mmol/L)干预高糖处理MCF-7细胞,结果显示:p-AMPKα1/2蛋白灰度值分别为0.26、0.49、0.50、0.51、0.53,与对照组相比,在二甲双胍浓度为2.5、5、10和20 mmol/L处理组中表达差异有统计学意义(P=0.036、0.029、0.001、0.000);AMPKα1蛋白灰度值分别为0.30、0.41、0.52、0.53、0.56,与对照组相比,在二甲双胍浓度为5、10和20 mmol/L处理组中差异有统计学意义(P=0.003、0.002、0.002),见图 2;AMPKα1 mRNA灰度值分别为0.24、0.33、0.34、0.42、0.53,与对照组相比,在二甲双胍浓度为2.5、5、10和20 mmol/L处理组中差异有统计学意义(P=0.012、0.007、0.005、0.000),见图 3;且AMPKα1和p-AMPKα1/2的蛋白表达、AMPKα1 mRNA的表达与二甲双胍浓度有显著正相关性(r=0.608、0.622、 0.905,P均<0.05),见图 4。
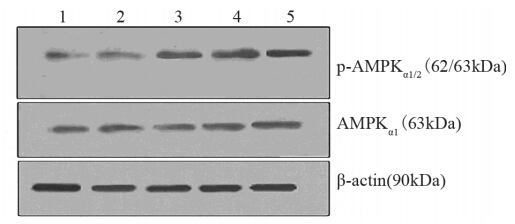 |
| 1: 0mmol/L metformin (control); 2: 2.5mmol/L metformin; 3: 5mmol/L metformin; 4: 10mmol/L metformin; 5: 20mmol/L metformin图 2 Western blot检测高糖条件下AMPKα1及p-AMPKα1/2 蛋白表达水平Figure 2 The expression of AMPKα1andp-AMPKα1/2 protein in MCF-7 cells in high glucose group detected by Western blot |
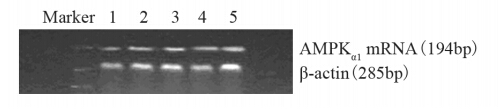 |
| 1: 0mmol/L metformin (control); 2: 2.5mmol/L metformin; 3: 5mmol/L metformin; 4: 10mmol/L metformin; 5: 20mmol/L metformin图 3 RT-PCR检测高糖作用条件下AMPKα1 mRNA表达水平Figure 3 The expression of AMPKα1mRNA in MCF-7 cells in high glucose group (20mmol/L glucose) detected by RT-PCR |
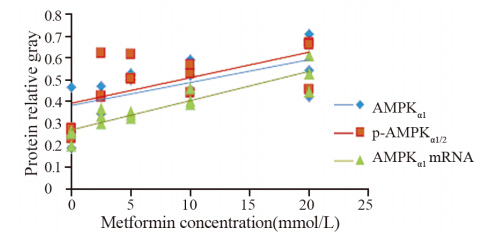 |
| AMPK protein expression had significant correlation with metformin concentration(r=0.608,P=0.018); p-AMPKα1/2 protein expression had significant correlation with metformin concentration(r=0.622 P=0.013); AMPKα1mRNA expression had significant correlation with metformin concentration(r=0.905,P=0.000)图 4 高糖组中AMPK和不同浓度二甲双胍的相关性Figure 4 The correlation between AMPK expression and metformin at different concentration in high glucose group |
AMPKα1、p-AMPKα1/2蛋白及AMPKα1 mRNA表达的影响
用不同浓度二甲双胍(0、2.5、5、10和20 mmol/L)干预高胰岛素处理MCF-7细胞,结果显示:AMPKα1蛋白灰度值分别为0.52、0.62、0.62、0.81、0.86,与对照组相比,在二甲双胍浓度为10和20 mmol/L处理组中差异有统计学意义(P=0.002,0.001);p-AMPKα1/2蛋白灰度值分别为0.34、0.53、0.54、0.67、0.67,与对照组相比,在二甲双胍浓度为2.5、5、10和20 mmol/L处理组中差异有统计学意义(P=0.001、0.000、0.000、0.000),见图 5;AMPKα1 mRNA灰度值分别为0.13、0.34、0.43、0.58、0.72,与对照组相比,在二甲双胍浓度为2.5、5、10和20 mmol/L处理组中差异有统计学意义(P=0.049、0.003、0.000、0.000),见图 6;AMPKα1和p-AMPKα1/2蛋白表达、AMPKα1 mRNA表达与二甲双胍浓度有显著正相关性(r=0.815、0.730,0.912,P均<0.01),见图 7。
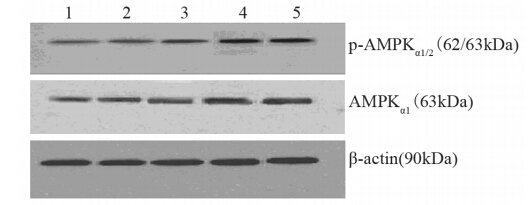 |
| 1: 0mmol/L metformin(control); 2: 2.5mmol/L metformin; 3: 5mmol/L metformin; 4: 10mmol/L metformin; 5: 20mmol/L metformin 图 5 Western blot检测高胰岛素作用下AMPKα1及p-AMPKα1/2 蛋白表达水平Figure 5 The expression of AMPKα1andp-AMPKα1/2 protein in MCF-7 cells in high insulin group detected by Western blot |
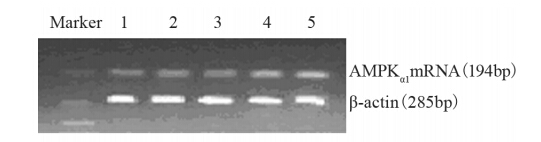 |
| 1: 0mmol/L metformin(control); 2: 2.5mmol/L metformin; 3: 5mmol/L metformin; 4: 10mmol/L metformin; 5: 20mmol/L metformin图 6 RT-PCR检测高胰岛素作用下AMPKα1 mRNA表达水 平Figure 6 The expression of AMPKα1mRNA in MCF-7 cells in high insulin group detected by RT-PCR |
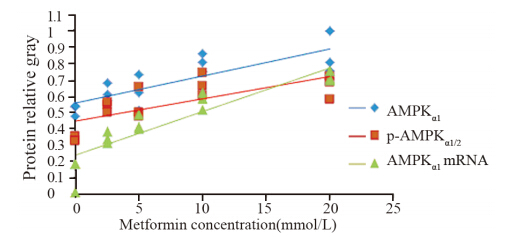 |
| AMPK protein expression had significant correlation with metformin concentration(r=0.815,P=0.000). p-AMPKα1/2 protein expression had significant correlation with metformin concentration(r=0.730, P=0.002). AMPKα1mRNA expression had significant correlation with metformin concentration(r=0.912,P=0.000)图 7 高胰岛素组中AMPK和不同浓度二甲双胍的相关性Figure 7 The correlation between AMPK expression and metformin at different concentration in high insulin group |
与对照组相比,AMPKα1蛋白灰度值分别为0.34、0.35、0.35、0.46、0.51,且在二甲双胍浓度为10和20 mmol/L处理组中差异有统计学意义(P=0.030、0.005);p-AMPKα1/2 蛋白灰度值分别为0.23、0.35、0.40、0.34、0.43,且在二甲双胍浓度为5、10和20 mmol/L处理组中差异有统计学意义(P=0.004、0.000、0.008),见图 8;AMPKα1 mRNA的灰度值分别为0.37、0.45、0.48、0.53、0.74,表达在二甲双胍浓度为2.5、5、10和20 mM处理组中差异有统计学意义(P=0.039、0.029、0.004、0.000),见图 9;AMPKα1和p-AMPKα1/2蛋白表达、AMPKα1 mRNA表达与二甲双胍浓度有显著正相关性(r=0.789、0.651、0.911,P均<0.01),见图 10。
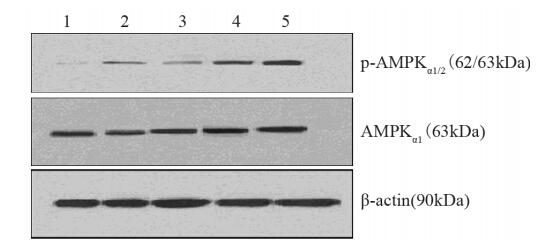 |
| 1: 0mmol/L metformin(control); 2: 2.5mmol/L metformin; 3: 5mmol/L metformin; 4: 10mmol/L metformin; 5: 20mmol/L metformin图 8 Western blot检测高糖+高胰岛素组不同浓度二甲双胍作用下AMPKα1及p-AMPKα1/2蛋白表达水平Figure 8 The expression of AMPKα1 and p-AMPKα1/2 protein in MCF-7 cells in high glucose plus insulin group detected by Western blot |
 |
| 1: 0mmol/L(control) metformin; 2: 2.5mmol/L metformin; 3: 5mmol/L metformin; 4: 10mmol/L metformin; 5: 20mmol/L metformin图 9 RT-PCR检测高糖+高胰岛素组不同浓度二甲双胍作用下AMPKα1mRNA表达水平Figure 9 The expression of AMPKα1mRNA in MCF-7 cells in high glucose plus high insulin group detected by RT-PCR |
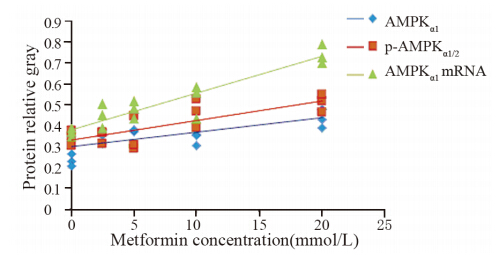 |
| AMPK expression had significant correlation with metformin concentration(r=0.789,P=0.000). p-AMPKα1/2 expression had significant correlation with metformin concentration(r=0.651, P=0.009). AMPKα1mRNA expression had significant correlation with metformin concentration (r=0.911,P=0.000)图 10 高糖+高胰岛素组中AMPK和不同浓度二甲双胍的相关性 Figure 10 The correlation between AMPK expression and metformin at different concentration in high glucose plus insulin group |
高糖+高胰岛素共同作用72h后,高糖组中G1期乳腺癌MCF- 7细胞所占百分比由(76.40±0.2)%下降至(74.6±0.4)%,差异有统计学意义(P=0.042),见图 11。
糖组中G1期乳腺癌MCF-7细胞所占百分比由(71.75±0.15)%增加至(76.80±0.5)%,且差异有统计学意义(P=0.0004)。高胰岛素组中G1期乳腺癌MCF-7所占百分比由(68.86±1.64)%增加至(73.63±1.02)%,且差异有统计学意义(P=0.022)。高糖和高胰岛素组中G1期所占百分比由(67.20±1.33)%增加至(74.53±1.84)%,且差异有统计学意义(P=0.030),见图 12。
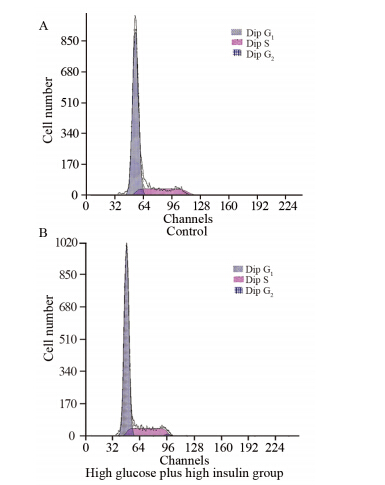 |
| 图 11 高糖+高胰岛素对乳腺癌MCF-7细胞周期的影响Figure 11 The effects of high glucose plus insulin on cell cycle of MCF-7 cells detected by flow cytometry |
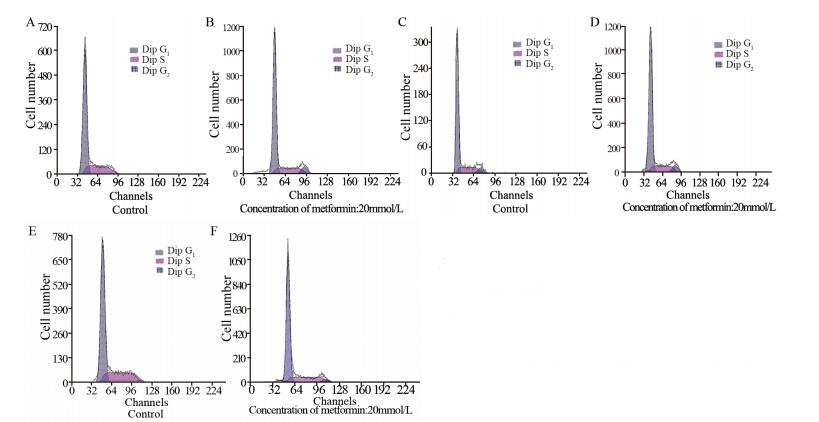 |
| A,B: high glucose group; C,D: high insulin group; E,F: high glucose plus high insulin group 图 12 二甲双胍对高糖和(或)高胰岛素条件下乳腺癌MCF -7细胞周期的影响Figure 12 The effect of metformin on cell cycle of MCF-7 cells treated with high glucose and (or) high insulin detected by flow cytometry |
糖尿病与肿瘤为影响人类健康的两大杀手,2型糖尿病患者可能同时有高糖、高胰岛素及高糖和高胰岛素并存状态,人们普遍认为高血糖可使肿瘤的发生率增高[5, 6],同时也有研究表明高胰岛素水平的绝经后非糖尿病女性患者患乳腺癌的风险较高,提示高胰岛素与乳腺癌的发生有关[7],本实验结果提示在高糖和高胰岛素共同作用条件下,AMPKα1蛋白表达水平降低,因此AMPK有可能在糖尿病和肿瘤中起到一定连接作用。
二甲双胍是一种广泛应用于2型糖尿病的口服药物,国外早有研究证实2型糖尿病为乳腺癌患者预后的不良因素,二甲双胍的应用可以使患者得到更长的生存期和更少的远处转移。此外,Bayraktar等[8]通过回顾性总结1 448例病例,结果提示二甲双胍可以增加乳腺癌患者的化疗缓解率。这些研究均提示二甲双胍具有一定抗肿瘤作用。人们普遍认为二甲双胍即可通过激活LKB1进而激活AMPK发挥降糖作用[9]。
LKB1是一种AMPK上游蛋白激酶,是人体重要的肿瘤抑制因子[10],在肿瘤的发生、发展中起着重要作用,同样AMPK还可以激活下游因子mTOR,mTOR主要通过对蛋白的调节发挥对细胞的生长产生影响,此外,因肿瘤细胞的能量代谢较正常细胞发生变化,主要以糖酵解为细胞的主要能量来源,而非线粒体的氧化磷酸化,这称为Warburg效应。最新研究发现,AMPK的激活可以抑制这种效应,影响肿瘤细胞能量生成,进而抑制肿瘤细胞的生长[11]。有关于AMPK抑制肿瘤的其他机制有待于进一步研究。
本实验采用Western blot及RT-PCR方法检测AMPK表达水平进而反映其对肿瘤细胞的影响,因AMPK分为不同亚基及亚型,不同亚型在不同组织中表达的量有所不同,我们选取了在乳腺癌MCF-7细胞中含量最多的催化亚基α1亚型作为检测对象,用以了解AMPK在不同条件下的表达水平。
本实验结果显示,高糖+高胰岛素均可使AMPK蛋白表达水平有所降低,提示AMPK可能在糖尿病及肿瘤的链接中起到一定作用,同样,在高糖和(或)高胰岛素作用情况下,应用不同浓度二甲双胍进行干预,AMPKα1及p-AMPKα1的蛋白水平及AMPKα1 mRNA表达水平均有不同程度的升高,提示二甲双胍在上述三种实验条件下均可以激活AMPK,进而在糖尿病的不同阶段均对AMPK起到调节作用,从而起到一定肿瘤抑制作用,这种作用强度与二甲双胍浓度有一定相关性。
细胞周期包含分裂间期G1期、S期、G2期和分裂期M期,人们发现在肿瘤细胞中G1期会发生改变,导致肿瘤细胞的增殖。本实验结果提示高糖+高胰岛素共同作用下,乳腺癌MCF-7细胞G1期数量较对照组有所下降,这可能提示高糖+高胰岛素同样可以通过控制细胞周期对肿瘤细胞的发生、发展起到调控作用。同样,Alimova等[12]将乳腺癌MCF-7细胞培养于不同浓度二甲双胍中24 h,结果提示二甲双胍干预后的细胞停滞于G1期,本次实验发现20 mmol/L的二甲双胍作用72 h后乳腺癌细胞株MCF-7细胞周期G1期的比例增高,S期和G2期的细胞比例减少,与之前的相关实验结果一致,提示二甲双胍是通过阻滞细胞于G1期来抑制细胞增长。关于高糖+高胰岛素及二甲双胍调控细胞周期的可能机制尚不十分明确,但可能与细胞周期G1期密切相关的Cyclin D1和E2F1的表达相关[13]。Cyclin D1和E2F1的表达在多种肿瘤细胞中都表达异常。
然而,在临床应用中,二甲双胍的血药浓度通常(每天0.5~3 g)在10~25 μmol/L之间,远小于本次实验应用的二甲双胍浓度。以往的体外实验发现,低浓度二甲双胍对低糖条件下的细胞生长及AMPK表达水平并无影响,当二甲双胍浓度大于10 mmol/L时AMPK表达才会有所升高[14],同样国外研究也发现,二甲双胍短期应用于乳腺癌患者并未表现出明显益处,只有在长期持续应用后才显现出来,多数患者要用药达5~10年[15]。因此关于二甲双胍应用于肿瘤临床治疗仍需进一步研究。
| [1] | Vigneri P, Frasca F, Sciacca L, et al. Diabetes and cancer[J]. Endocr Relat Cancer, 2009, 16(4): 1103-23. |
| [2] | Onitilo AA, Engel JM, Glurich I, et al. Diabetes and cancer I: risk, survival, and implications for screening[J]. Cancer Causes Control, 2012, 23(6): 967-81. |
| [3] | Gill RK, Yang SH, Meerzaman D, et al. Frequent homozygous deletion of the LKB1/STK11 gene in non-small cell lung cancer.[J] Oncogene, 2011, 30(35): 3784-91. |
| [4] | Martin M, Marais R. Metformin: A diabetes drug for cancer, or a cancer drug for diabetics? [J]. J Clin Oncol, 2012, 30(21): 2698-700. |
| [5] | Rapp K, Schroeder J, Klenk J, et al. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria[J]. Diabetologia, 2006, 49(5): 945-52. |
| [6] | Muti P, Quattrin T, Grant BJ, et al. Fasting glucose is a risk factor for breast cancer: a prospective study[J]. Cancer Epidemiol Biomarkers Prev, 2002, 11(11): 1361-8. |
| [7] | Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women[J]. J Natl Cancer Inst, 2009, 101(1): 48-60. |
| [8] | Bayraktar S, Hernadez-Aya LF, Lei X, et al. Effect of metformin on survival outcomes in diabetic patients with triple receptornegative breast cancer[J]. Cancer, 2012, 118(5): 1202-11. |
| [9] | Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells[J]. Cancer Res, 2007, 67(22): 10804-12. |
| [10] | Collins SP, Reoma JL, Gamm DM, et al. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vitro[J]. Biochem J, 2000, 345 (3): 673-80. |
| [11] | Faubert B, Boily G, Izreig S, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo[J]. Cell Metab, 2013, 17(1): 113-24. |
| [12] | Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro[J]. Cell Cycle, 2009, 8(6): 909-15. |
| [13] | Yu Z, Wang L, Wang C, et al. Cyclin D1 induction of Dicer governs microRNA processing and expression in breast cancer[J]. Nat Commun, 2013, 4: 2812. |
| [14] | Rocha GZ, Dias MM, Ropelle ER, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth[J]. Clin Cancer Res, 2011, 17(12): 3993-4005. |
| [15] | Bodmer M, Meier C, Kr?henbühl S, et al. Long-term metformin use is associated with decreased risk of breast cancer[J]. Diabetes Care, 2010, 33(6): 1304-8. |
 2015, Vol. 42
2015, Vol. 42


