文章信息
- 李文雯,张琳丽,胡国清. 2015
- LI Wenwen, ZHANG Linli, HU Guoqing. 2015
- 转录因子Snail对食管癌Eca-109细胞侵袭和迁移的影响
- Effect of Transcription Factor Snail on Invasion and Migration of Esophageal Carcinoma Cell Line Eca-109
- 肿瘤防治研究, 2015, 42(04): 319-323
- Cancer Research on Prevention and Treatment, 2015, 42(04): 319-323
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2015.04.001
-
文章历史
- 收稿日期:2014-09-10
- 修回日期:2014-12-16
食管癌是我国常见的消化道恶性肿瘤之一,其死亡率居全国恶性肿瘤第四位[1]。随着多学科综合治疗的发展,食管癌的预后得到一定的改善,但远处转移仍然是导致患者死亡的主要原因之一[2]。因此寻找调控肿瘤侵袭转移的关键靶点,研究针对靶基因的治疗药物,将对提高食管癌的疗效具有重要的临床意义。上皮间质转化(epithelial-mesenchymal transition,EMT)是指上皮细胞失去细胞间的连接和极性,获得类似纤维母细胞样的间质细胞特性的过程,它不仅是多细胞生物胚胎发育与器官形成的基础,而且与肿瘤的发生、侵袭和转移有着密切联系[3]。多种转录因子参与调控上皮间质转化的过程,其中的关键基因Snail1是Snail超家族的一员,它通过特定的生物学作用调控EMT进而促进肿瘤的发展[4]。其异常表达也与包括食管癌在内的多种肿瘤的预后密切相关[5, 6]。本研究通过慢病毒转染构建过表达Snail的食管鳞癌细胞株,在体外水平验证其在调控肿瘤细胞侵袭迁移中的作用,并初步阐明其调控机制。1 材料和方法1.1 实验材料 1.1.1 细胞
人食管高分化鳞癌Eca-109细胞株购自武汉大学细胞库,由同济医院肿瘤中心实验室保存。1.1.2 主要试剂
RPMI1640培养液购自美国HyClone公司;胎牛血清(fetal bovine serum,FBS)购自杭州四季青公司;BCA蛋白定量试剂盒为上海碧云天生物技术研究所产品;Snail、E-cadherin兔抗人单克隆抗体均购自美国Cell Signaling Technology公司;Vimentin、MMP-2兔抗人单克隆抗体均购自美国Epitomics公司;人Snail基因过表达慢病毒(GV166:Ubi-MCS-3FLAG-IRES-puro)、阴性对照慢病毒(GV166-null)均购自上海吉凯基因化学技术有限公司。1.1.3 引物序列设计与合成
Real-time PCR引物序列由苏州金唯智生物科技有限公司合成。1.2 实验方法1.2.1 细胞培养
Eca-109细胞的常规培养采用含10%FBS的RPMI1640培养液,在37℃、5%CO2培养箱中培养。1.2.2 慢病毒转染Eca-109细胞
将对数生长期的Eca-109细胞制备成单细胞悬液,按9×104个/孔接种于12孔板中,12 h后按复感染指数(multiplicity of infection,MOI)=50分别加入Snail过表达或对照空载(对照组)的病毒液。转染10 h后更换完全培养液,24 h后用含1 μg/ml嘌呤霉素的完全培养液培养48 h,筛选转染成功的Eca-109细胞,收集细胞,进一步鉴定。1.2.3 Real-time PCR检测
收集各组细胞,用TRIzol法提取细胞总RNA,TaKaRa公司反转录试剂盒合成cDNA,按SYBR Green试剂盒(Invitrogen公司)说明书于实时荧光定量PCR仪进行扩增。PCR引物:Snail引物:5’-TCGGAAGCCTAACTACAGCCA- 3’(上游);5’ - AGATGAGCATTGGCAGCGAG-3’(下游);GAPDH引物:5’-GGTCGGAGTCAACGGATTTG-3’(上游),5’-GGAAGATGGTGATGGGATTTC-3’(下游)。反应条件:(1) 95℃ 30 s;(2) 95℃8 s,60℃32 s,40个循环;(3) 95℃ 1 min,60℃ 30 s,95℃ 30 s。最后以2-ΔΔCT法计算基因相对表达量。1.2.4 Western blot实验
用RIPA细胞裂解液于冰上裂解细胞,裂解产物经离心、蛋白变性后,加入Loading buffer上样至SDS-PAGE胶电泳分离,PVDF膜恒流电转,5%脱脂奶粉封闭1 h,TweenPBS洗膜后加入一抗,4℃过夜孵育,再次洗膜后加入二抗,室温孵育1 h,Tween-PBS洗膜,ECL化学发光法显影,凝胶成像分析系统进行灰度扫描,计算各组条带的灰度值。实验选用GAPDH为内参对照。1.2.5 细胞划痕实验
取对数生长期Eca- 109-Snail、Eca-109-vc细胞分别接种于24孔板中培养至95%融合后,用10 μl无菌枪头垂直划出一条无细胞区,小心洗去漂浮细胞3遍后加入无血清培养液,放至37℃、5%CO2培养箱培养,分别在划痕0、24 h后于显微镜下观察并拍照。各实验组24 h细胞迁移距离(mm)= 0 h划痕宽度-24 h划痕宽度;24 h细胞迁移率=(0 h划痕宽度-24 h划痕宽度)/0 h划痕宽度×100%。1.2.6 Transwell实验
于Transwell板上室面铺ECM胶40 μl,5 h后,取对数生长期细胞,以每毫升1×105个密度接种于上室,下室加入含10%胎牛血清的完全培养液600 μl,于37℃、5%CO2培养箱培养24 h,再经甲醇固定,0.1%结晶紫染色后,用棉签刮除上室面ECM胶,于显微镜下随机选取5个视野(×200)观察并拍照,计数每个视野中穿过滤膜的细胞数。1.3 统计学方法
应用SPSS17.0统计学软件分析。均值检验采用student’s t test,P<0.05为差异有统计学意义。 2 结果2.1 稳定转染细胞株Eca-109-Snail和Eca-109-vc的建立
慢病毒转染后,嘌呤霉素分别筛选出稳定转染细胞株,经Real-time PCR及Western blot实验鉴定显示,转染过表达Snail病毒的食管癌细胞的Snail mRNA相对表达量为(39.45±0.75),显著高于Eca-109-vc组(0.71±0.14)及未转染组细胞(1.0±0.0),差异具有统计学意义(P=0.0043,P=0.0036),见图 1A。Snail蛋白在过表达组相对表达量为(0.81±0.05)显著高于Eca-109-vc组细胞(0.34±0.07)和未转染组细胞(0.16±0.04),差异具有统计学意义(P=0.0062,P=0.0013),见图 2。据此将转染过表达Snail病毒的食管癌细胞认定为稳定过表达Snail的Eca-109-Snail细胞,空载体转染组细胞为Eca-109-vc细胞。
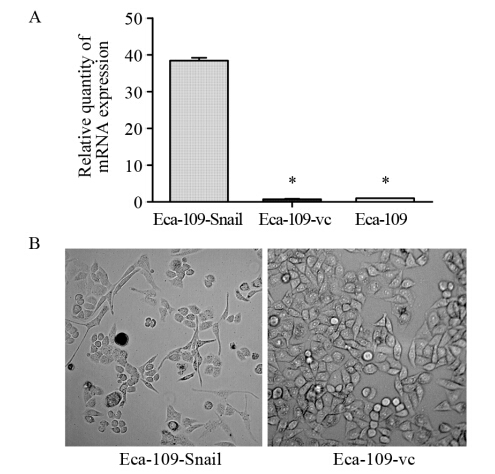 |
| *: P<0.05,compared with Eca-109-Snail cells A: expression levels of Snail mRNA detected by Real-time PCR; B: the presence of spindle-shaped cells with loss of polarity,increased intercellular separation,and pseudopodia were noted in Eca-109-Snail cells but not in Eca-109-vc cells (×200) 图 1 Snail过表达食管癌细胞株的建立及其EMT样表型的改变Figure 1 The establishment and EMT-like morphological changes of over-expressed Snail in esophageal carcinoma cell lines |
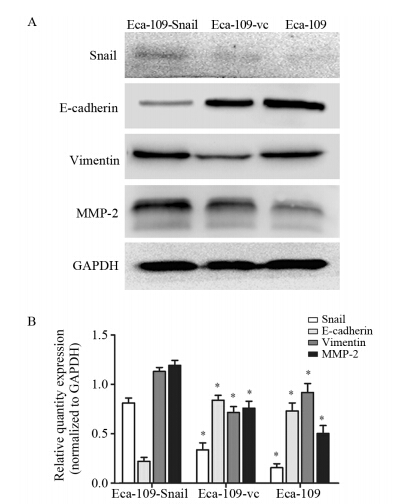 |
| *: P<0.05,compared with Eca-109-Snail 图 2 Western blot实验检测EMT相关蛋白的表达情况 Figure 2 Expression levels of EMT related protein detected by Western blot |
Snail过表达食管癌Eca-109-Snail细胞表现出成纤维细胞样的外形,细胞之间彼此分离,见图 1B。Western blot实验显示Eca-109-Snail细胞E-cadherin蛋白表达量(0.22±0.04)较空载体转染组Eca-109-vc细胞(0.84±0.05)以及对照组细胞(0.73±0.08)降低(P=0.0031;P=0.0010);Eca-109-Snail细胞中Vimentin蛋白表达量(1.13±0.04)较空载体转染组Eca-109-vc细胞(0.72±0.06)、对照组细胞(0 .8 9 ±0 .0 9)升高(P=0 .0 0 8 2; P=0.010);Snail过表达组细胞株MMP-2蛋白表达(1.19±0.05)均较空载体转染组(0.75±0.07)及对照组细胞(0.50±0.08)升高(P=0.0012; P=0.0043),见图 2,差异均具有统计学意义。提示Snail的高表达可以抑制E-cadherin蛋白的表达,上调Vimentin、MMP-2的表达。 2.3 Snail基因促进食管癌细胞的迁移
细胞划痕实验显示24 h后Eca-109-Snail及Eca-109-vc的细胞划痕愈合率分别为(62.51±5.73)%和(14.66±2.82)%,过表达组细胞可见明显的细胞迁移效应,与对照组比较差异具有统计学意义(P=0.0002),见图 3。结果提示Snail基因参与诱导食管癌细胞迁移能力增强。
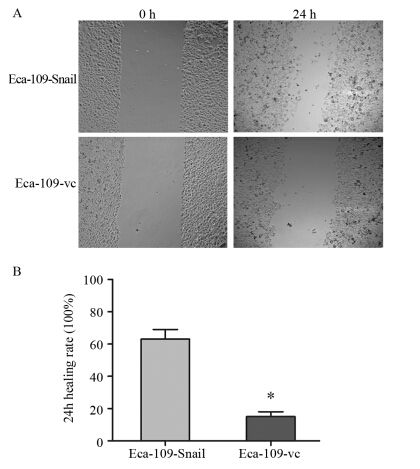 |
| *: P<0.05,compared with Eca-109-Snail 图 3 细胞划痕实验检测食管癌Eca-109细胞株的迁移能力(×100)Figure 3 Effect of Snail on migration of Eca-109 cell lines detected by Wound healing assay(×100) |
Transwell侵袭实验中,Snail过表达组细胞穿过上室ECM胶及聚碳酸酯膜的数量为(7 4 .0 ±8.1)个/HP,空载对照组细胞数为(35.0±5.2)个/HP,Snail过表达组细胞侵袭能力明显高于空载对照组细胞,两组比较差异有统计学意义(P=0.0026),见图 4。提示Snail基因的过表达诱导食管癌细胞侵袭能力增强。
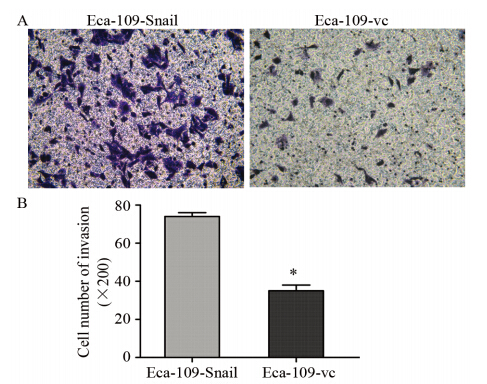 |
| *:P<0.05,compared with Eca-109-Snail图 4 Transwell实验检测食管癌Eca-109细胞侵袭能力(×200) Figure 4 Effect of Snail on invasion of Eca-109 cell lines detected by Transwell assay(×200) |
食管癌是我国恶性程度较高且预后较差的恶性肿瘤之一[1]。由于起病隐匿,早期症状不典型,恶性程度高,70%的患者表现出临床症状时已出现转移[7]。因此研究食管癌侵袭转移的机制并寻找新的治疗靶点仍是该领域的科研热点。
肿瘤的侵袭转移是一个复杂的过程,包括降低细胞之间的黏附、降解细胞外基质以及促进细胞运动等过程[8]。近年来已有越来越多的研究表明,在多种上皮来源的恶性肿瘤的侵袭转移行为中存在上皮间质转化的过程,EMT在肿瘤的恶性进展以及浸润、转移中发挥重要作用[9, 10]。目前普遍认为E-cadherin的下调或丢失是EMT的重要分子标志,致使肿瘤细胞的细胞极性和细胞间的黏附作用减弱或丧失,同时使上皮细胞获得间充质细胞特点,如细胞骨架的重建、细胞外基质的合成增加、上调间质相关蛋白等[11]。大量研究表明,锌指转录因子Snail是调控EMT的关键基因,通过与E-cadherin启动子上的E-box作用元件结合从而抑制E-cadherin的转录[12]。对食管癌病例进行分析发现:Snail高表达的患者总生存相对较差;Snail在食管癌中的表达随肿瘤分化程度的降低而升高,Snail阳性的肿瘤组织浸润深度更深,更容易出现淋巴结转移及远处转移[13, 14]。
本实验利用慢病毒作为有效的载体,成功构建过表达Snail的食管癌细胞株Eca-109-Snail,通过特异性上调Snail表达后,观察Snail基因在调控食管癌细胞发生EMT,并进一步诱导侵袭转移中的作用。结果显示,过表达组细胞之间彼此分离,表现出成纤维细胞样形态。同时,Snail的上调抑制了上皮细胞标志物E-cadherin的表达,诱导间质细胞标志蛋白Vimentin的表达升高,通过这一系列作用,最终促进食管癌上皮细胞发生间质表型转化。此外,基质金属蛋白酶(matrix metalloproteinases,MMPs)是一类锌依赖的蛋白水解酶家族,它的活化是诱导肿瘤浸润和转移的重要步骤。其家族成员MMP-2能够降解细胞外基质中的主要成分—Ⅳ型胶原,在肿瘤侵袭转移中发挥关键作用[15]。本研究发现Snail过表达组食管癌细胞MMP-2蛋白表达升高,提示Snail参与对MMP-2的调节,促进肿瘤细胞对细胞外基质成分的降解,使食管鳞癌上皮细胞侵袭、迁移能力显著增强。
本研究证实了食管癌Eca-109细胞中Snail基因为调控侵袭和迁移的重要因子之一,通过调控EMT相关蛋白表达,介导食管癌细胞丧失细胞间连接,诱导食管癌细胞获得降解细胞外基质并向远处运动的能力,最终促进食管癌细胞的侵袭和迁移。以往研究也证明了利用siRNA沉默Snail基因的表达可以显著降低食管癌细胞EC9706的体外侵袭能力[16]。因此Snail基因作为EMT的重要调控靶点,对于肿瘤的发生发展起着关键作用。然而在其诱导肿瘤细胞上皮间质转化,并进一步调控侵袭转移的过程,具体是通过何种信号通路和细胞因子协同作用的,仍然有待进一步探索。本研究为深入研究Snail在食管癌侵袭转移中的作用机制奠定了基础,为针对EMT的肿瘤治疗提供了依据。
| [1] | Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010[J]. Chin J Cancer Res, 2014, 26(1): 48-58. |
| [2] | Enzinger PC, Mayer RJ. Esophageal cancer[J]. N Engl J Med, 2003, 349(23): 2241-52. |
| [3] | Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression[J]. J Mammary Gland Biol Neoplasia, 2010, 15(2): 117-34. |
| [4] | Nieto MA. The snail superfamily of zinc-finger transcription factors[J]. Nat Rev Mol Cell Biol, 2002, 3(3): 155-66. |
| [5] | Fan TL, Hou GQ, Xi Y, et al. Expression of zinc finger transcription factor Snail and E-cadherin in esophageal cancer and its significance[J]. Zhong Liu Fang Zhi Yan Jiu, 2010, 37(8): 918-21. [范天黎, 侯桂琴, 席宇, 等. 锌指转录因子Snail及E-钙黏附素在食管鳞癌中的表达及意义[J]. 肿瘤防治研究, 2010, 37(8): 918-21.] |
| [6] | Kroepil F, Fluegen G, Vallbohmer D, et al. Snail1 expression in colorectal cancer and its correlation with clinical and pathological parameters[J]. BMC Cancer, 2013, 13: 145. |
| [7] | Crosby TD, Evans M. Resectable oesophageal cancer: a practice guideline update. Practice changing or supporting the status quo?[J]. Clin Oncol (R Coll Radiol), 2010, 22(4): 247-9. |
| [8] | Sethi N, Kang Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies[J]. Nat Rev Cancer, 2011, 11(10): 735-48. |
| [9] | Guarino M, Tosoni A, Nebuloni M. Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition[J]. Hum Pathol, 2009, 40(10): 1365-76. |
| [10] | Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression[J]. J Cell Physiol, 2007, 213(2): 374-83. |
| [11] | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition[J]. Nat Rev Mol Cell Biol, 2014, 15(3): 178-96. |
| [12] | Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression:an alliance against the epithelial phenotype?[J]. Nat Rev Cancer, 2007, 7(6): 415-28. |
| [13] | Kuo KT, Chou TY, Hsu HS, et al. Prognostic significance of NBS1 and Snail expression in esophageal squamous cell carcinoma[J]. Ann Surg Oncol, 2012, 19 Suppl 3: S549-57. |
| [14] | Natsugoe S, Uchikado Y, Okumura H, et al. Snail plays a key role in E-cadherin-preserved esophageal squamous cell carcinoma[J]. Oncol Rep, 2007, 17(3): 517-23. |
| [15] | Kallakury BV, Karikehalli S, Ross JS, et al. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma[J]. Clin Cancer Res, 2001, 7(10): 3113-9. |
| [16] | Lu QA, Kand LL. The relativity between Snail gene and invasion and migration ability of esophageal carcinoma cell [J]. Zhongguo Yi Yao Dao Bao, 2011, 8(26): 24-6. [鲁秦安, 康龙丽. Snail基因与食管癌细胞侵袭迁移能力的相关性分析[J]. 中国医药导报, 2011, 8(26): 24-6.] |
 2015, Vol. 42
2015, Vol. 42


