文章信息
- 吴建忠, 陈琳, 刘晓安, 高泉根,黄维贤.
- WU Jianzhong, CHEN Lin, LIU Xiaoan, GAO Quangen, HUANG Weixian.
- 肿瘤浸润性淋巴细胞比例在乳腺癌新辅助化疗中的意义
- Tumor-infiltrating Lymphocytes Predict Response to Neoadjuvant Chemotherapy in Patients with Breast Carcinoma
- 肿瘤防治研究, 2015, 42(11): 1114-1118
- Cancer Research on Prevention and Treatment, 2015, 42(11): 1114-1118
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2015.11.012
-
文章历史
- 收稿日期: 2015-01-19
- 修回日期: 2015-03-17
2. 210029 南京,南京医科大学第一附属医院普外科
2. Department of General Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China
乳腺癌的发生、发展由一系列因素所决定,其中包括肿瘤细胞、间质细胞和宿主的炎症细胞之间的相互作用。目前国外学者研究表明在乳腺癌组织中出现较多淋巴细胞可预示较好的治疗效果[1, 2, 3]及免疫系统也参与了杀灭肿瘤细胞及控制肿瘤生长的过程[4, 5]。最近,又有学者提出肿瘤组织中提前的免疫反应可以提高化疗药物的疗效性[6, 7]。尽管已有大量的证据,但很难有具体参数来将肿瘤基础免疫应用于临床试验。
新辅助化疗虽有很高的有效率,约70%~90%[8, 9]。但对于化疗无效的乳腺癌患者,术前的化疗只会增加患者的痛苦和延误手术时机。通过空心针穿刺取材,分析肿瘤的生物因素,如能从中找出某些指标及参数,为那些化疗敏感的患者提供参考依据,也将是乳腺癌治疗的一个新进展。
本研究通过对59例新辅助化疗患者资料进行回顾性研究,测定肿瘤淋巴细胞的比例,推断肿瘤浸润性淋巴细胞预测新辅助化疗的有效性。
1 材料与方法 1.1 标本来源收集2010年12月—2013年11月期间59例女性乳腺癌患者,年龄31~71岁,中位年龄51岁,其中≤35岁的9例,以上患者均未接受过化疗、内分泌治疗及放疗。组织学类型:浸润性导管癌51例、浸润性小叶癌5例、腋窝肿块浸润性癌2例(其中1例术后病理提示导管癌)、黏液癌1例。雌激素受体表达阳性27例、阴性32例;孕激素受体表达阳性20例、阴性39例;人EGF受体 2基因(HER2)表达阳性34例、阴性25例;临床TNM分期:Ⅱ期33例、Ⅲ期26例。化疗方案:以蒽环类药物为基础的19例、以蒽环类加紫杉醇类为基础的40例,术前进行3~4个疗程。
1.2 方法 1.2.1 浸润性淋巴细胞判定浸润性淋巴细胞分为肿瘤内淋巴细胞(intratumoral lymphocyte, iTu-Ly)及间质淋巴细胞(stromal lymphocyte, str-Ly),见图 1。肿瘤内淋巴细胞定义为癌巢内或直接接触癌细胞的淋巴细胞,可报告为含淋巴细胞浸润的癌巢比例。间质淋巴细胞则是指肿瘤间质中含淋巴细胞浸润但未直接接触癌细胞的区域,可用所占面积百分比计量。浸润性淋巴细胞比例评分[10, 11, 12, 13]:间质淋巴细胞(str-Ly)比例评分分为0~3级,0级:0;1级:≤10%;2级:>10%~50%;3级:>50%。肿瘤内淋巴细胞(iTu-Ly)比例评分分为0~3,0级:0;1级:<10%;2级:≥10%~60%;3级:>60%。取经苏木精-伊红(hematoxylin and eosin stain, HE)染色的乳腺癌石蜡片,每张切片在光学显微镜下随机取10个视野,统计浸润性淋巴细胞比例,然后测算平均数。分别计算每个患者的肿瘤内淋巴细胞比例评分与间质淋巴细胞比例评分的总和,3~6级定义为高比例淋巴细胞,0~2级定义为低比例淋巴细胞,见图 2。本研究中发现术后只有7%化疗无效,癌巢结构无明显改变,其余患者经化疗后,癌巢结构破坏,肿瘤细胞坏死,被大量淋巴细胞浸润[14, 15],无法测定肿瘤内淋巴细胞的比例。但间质内淋巴细胞数量明显增多,所测的值较化疗前升高,我们把每提高>1级的患者定义为间质内淋巴细胞比例增强,≤1级的定义为间质内淋巴细胞比例无变化。
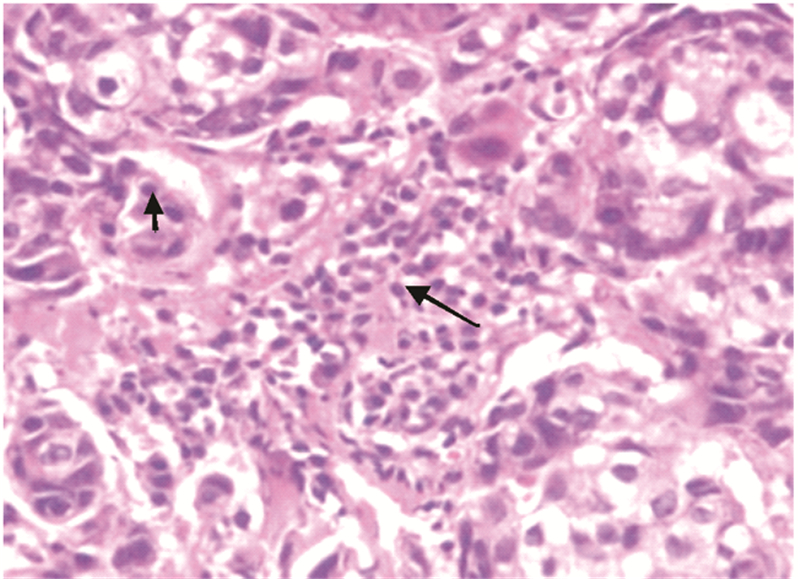
|
| The short arrow represented intratumoral lymphocytes and the long arrow represented stromal lymphocytes 图 1 乳腺癌组织学观察 (HE ×400) Figure 1 Histological observation of breast carcinoma tissues (HE ×400) |
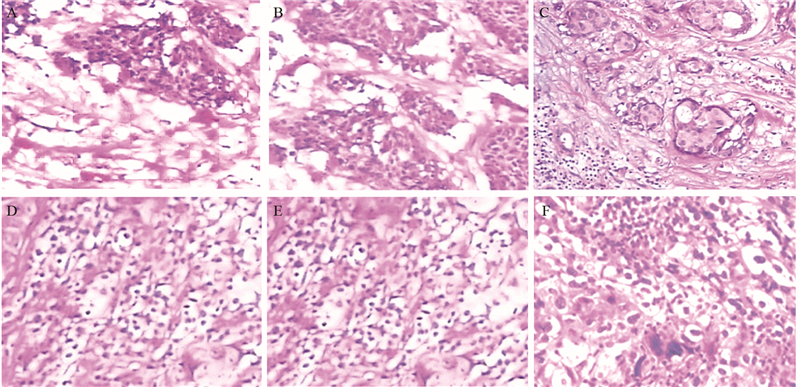
|
| A: Grade 0; B: Grade 1; C: Grade 2; D: Grade 3; E: Grade 4; F: Grade 5 and 6 图 2 乳腺癌浸润性淋巴细胞比例评分组织学观察 (HE ×200) Figure 2 Histological observation of tumor-infiltrating lymphocyte count score in breast carcinoma tissues (HE ×200) |
参考Miller和Payne分级方法[16]:1级:浸润癌组织的数量无变化;2级:浸润癌组织的数量减少比例小于30%;3级:浸润癌组织的数量明显减少,比例介于30%~90% ;4级:浸润癌组织的数量减少大于90%,仅有少数残余的癌细胞散在分布;5级(PCR):所有切片均无浸润性癌残存,指乳腺、腋窝组织中均无浸润性癌,可以有残存的导管内癌成分。病理疗效评价由两名病理科医师独立盲法阅片完成。根据MP疗效评价标准:1~2级评定为化疗无效,3~5级评定为化疗有效。
1.3 统计学方法采用SPSS10.0统计软件进行统计分析。计数资料采用卡方检验,P<0.05为差异具有统计学意义。
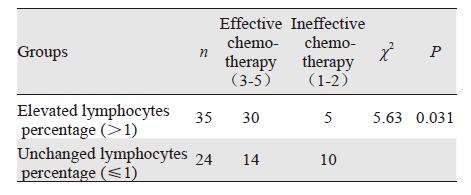
2 结果59例乳腺癌中,化疗有效率为44/59(74.5%),其中1级4例(7%),2级11例(18.6%),3级23例(39.0%),4级11例(18.6%),5级10例(17%),见图 1~图 3。高比例浸润淋巴细胞组的新辅助化疗有效率明显高于低比例组,差异具有统计学意义(P=0.001),见表 1。化疗后间质淋巴细胞比例明显增强组化疗有效率高于比例无变化组,差异具有统计学意义(P=0.031),见表 2。
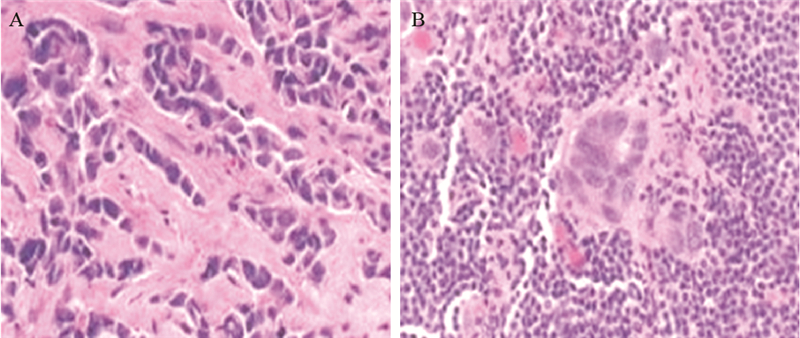
|
| A: before chemotherapy; B: after chemotherapy 图 3 乳腺癌组织间质内淋巴细胞变化 (HE ×200) Figure 3 Changes of stromal lymphocytes in breast carcinoma tissues (HE ×200) |
 |
 |
新辅助化疗在乳腺癌治疗中的许多优势已被普遍接受。它可以降低肿瘤的分期,使肿瘤体积缩小或消失,增加了手术切除或保乳手术的成功率;评价肿瘤对新辅助化疗方案的敏感度等。目前衡量新辅助化疗最有效的指标是病理完全缓解(PCR)[17]。大量研究显示乳腺癌的治疗过程中,新辅助化疗的总体有效率达70%[8, 9]以上,其中10%~25%[8, 9]达PCR。本研究中74.5%达到病理缓解,其中17%PCR,7%完全无反应。
目前研究表明在新辅助化疗过程中,免疫系统的参与也起了重要的作用,Zitvogel等[18]认为宿主的免疫系统参与能够提高化疗药物杀伤肿瘤细胞的能力。Denkert等[11]认为被化疗药物杀伤的肿瘤细胞释放出肿瘤相关性抗原,它们触发了免疫反应,大大提高了杀伤肿瘤细胞的能力。Hornychova等[19]认为化疗药物对肿瘤细胞的杀伤能力是有限的,但是它激发的免疫反应对肿瘤细胞的杀伤力将超过化疗药物本身的细胞毒性。淋巴细胞作为免疫细胞的一种,它在肿瘤治疗过程中的变化,也引起了广泛的关注。一些学者通过免疫组织化学技术[20]及细胞流量技术[21]测得了肿瘤淋巴细胞的亚型,另外在对乳腺癌的研究中表明肿瘤淋巴细胞和患者的预后存在着明显的正相关,此外,肿瘤淋巴细胞的浸润程度,尤其高比例的肿瘤内淋巴细胞浸润可以预示一个很好的治疗效果[22, 23],本研究结果和其一致(P=0.001)。乳腺髓样癌患者,即使ER(-)、较差的病理分级,经治疗后,患者还是能取得较好的预后,这可能和较其他乳腺癌类型拥有较高的浸润淋巴细胞比例相关[24, 25]。也有学者提出肿瘤浸润性淋巴细胞可作为评定化疗效果的一个独立预测因素[11,19] 。同时,经新辅助化疗后间质内淋巴细胞比例均有不同程度的提高,可能预示着免疫系统的主动参与及宿主清除癌细胞的能力增强[19],本研究表明化疗后间质内淋巴细胞比例增加明显者较轻度增强者可获得较高的病理缓解率(P=0.031)。结合我们对肿瘤内及间质内淋巴细胞的研究结果,推断如下:化疗并不只是单纯的杀灭肿瘤细胞,还可发挥免疫调节作用。化疗可诱导肿瘤细胞产生激发免疫应答的凋亡,使肿瘤抗原显露,从而有利于免疫细胞辨认肿瘤抗原,增强对肿瘤细胞的杀伤作用,因而化疗有可能通过触发抗肿瘤免疫来提高疗效。
最近研究表明,被某些化疗药物杀死的肿瘤细胞能激发T淋巴细胞的毒性反应,形成较为持久的抗肿瘤免疫,大大增加了杀灭肿瘤细胞的能力。因此有些化疗药物如紫杉醇,蒽环类药物在某种程度上被认为是一种免疫治疗药物[26, 27]。Carey临床试验表明以紫杉醇和蒽环类药物为基础的化疗方案优于单以蒽环类药物的化疗方案,蒽环类联合紫杉醇较单用蒽环类有较高的病理缓解率[28],本研究结果相同(P=0.012)。我们也推想联合使用此两种药物在抗肿瘤方面,除了细胞毒性作用外,在一定程度上激发了免疫系统来参与对肿瘤细胞的清除和对肿瘤生长的控制。
肿瘤细胞宿主的免疫系统对抗肿瘤药物的疗效起着非常重要的作用,如果药物要发挥最佳的作用必须有一个完整的免疫系统。抗肿瘤药物对肿瘤的作用也可以促进宿主自身的免疫的发展和作出适当的免疫反应。有时大剂量的糖皮质激素被用于化疗药物的不良反应如呕吐,因糖皮质激素是免疫抑制剂,这些药物的使用会抑制肿瘤药物的免疫辅助作用,同样,Parshad等[29]研究表明化疗过程中使用法莫替丁能提高化疗的疗效,这给临床用药做了提醒。
本研究表明肿瘤浸润淋巴细胞和化疗反应之间存在着较强的相关性,可望将它作为一个重要的参数来预测化疗效果。基于此研究及大量的文献资料,我们也提出是否化疗结合免疫治疗能取得更好的疗效。本研究的样本量较小,以后需进一步收集标本和周边单位合作,增大标本量,并应用免疫组织化学技术对肿瘤浸润性淋巴细胞进行分类,为浸润淋巴细胞可作为预测指标提供更多的理论依据。
| [1] | Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer[J]. N Engl J Med, 2005, 353(25): 2654-66. |
| [2] | DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response:crosstalk between adaptive and innate immune cells during breast cancer progression[J]. Breast Cancer Res, 2007, 9(4): 212. |
| [3] | Schmidt M, B?hm D, von T?rne C, et al. Thehumoral immune system has a key prognostic impact in node-negative breast cancer[J]. Cancer Res, 2008, 68(13): 5405-13. |
| [4] | Zitvogel L, Kroemer G. The immune response against dying tumor cells: Avoid disaster, achieve cure[J]. Cell Death Differ, 2008, 15(1): 1-2. |
| [5] | Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy[J]. Immunol Rev, 2007, 220: 47-59. |
| [6] | Apetoh L, Tesniere A, Ghiringhelli F, et al. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies[J]. Cancer Res, 2008, 68(11): 4026-30. |
| [7] | Zitvogel L, Apetoh L, Ghiringhelli F, et al. Immunological aspects of cancer chemotherapy[J]. Nat Rev Immunol, 2008, 8(1): 59-73. |
| [8] | Smith IC, Heys SD, Hutcheon AW, et al. Neoadjuvant chemotherapy in breast cancer: Significantly enhanced response with docetaxel[J]. J Clin Oncol, 2002, 20(6): 1456-66. |
| [9] | Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer[J]. J Clin Oncol, 1998, 16(8): 2672-85. |
| [10] | Black MM, Speer FD, Opler SR. Structural representations of tumor-host relationship in mammary carcinoma: Biologic and prognostic significance[J]. Am J Clin Pathol, 1956, 26(3): 250-65. |
| [11] | Denkert C, Loibl S, Noske A. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer[J]. J Clin Oncol, 2010, 28(1): 105-3. |
| [12] | Demaria S, Voim MD, Shapiro RL, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy[J].Clin Cancer Res, 2001, 7(10): 3025-30. |
| [13] | Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer[J]. Breast Cancer Res Treat, 2012, 132(3): 793-805. |
| [14] | Feng G, Kang H, Zhang Y, et al. Histopathological changes of breast cancer after neoadjuvant chemotherapy[J]. Shandong Yi Yao, 2009, 49(40), 22-4. [冯根, 康骅, 张雁, 等. 乳腺癌新辅助化疗前后病理组织学的变化[J]. 山东医药, 2009, 49(40): 22-4.] |
| [15] | Rajan R, Esteva FJ, Symmam WF. Pathologic changes in breast cancer following neoadjuvant chemotherapy: implications for the assessment of response[J]. Clin Breast Cancer, 2004, 5(3): 235-8. |
| [16] | Gong XY, Ding HY. Breast pathology[M]. Beijing: People’s Medical Publishing House, 2009: 506-7. [龚西騟, 丁华野. 乳腺病理学[M]. 北京: 人民卫生出版社, 2009: 506-7.] |
| [17] | Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27[J]. J Clin Oncol, 2008, 26(5): 778-85. |
| [18] | Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: Indispensable for therapeutic success?[J]. J Clin Invest, 2008, 118(6): 1991-2001. |
| [19] | Hornychova H, Melichar B, Tomsova M, et al. Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma[J]. Cancer Invest, 2008, 26(10): 1024-31. |
| [20] | Georgiannos SN, Renaut A, Goode AW, et al. The immunophenotype and activation status of the lymphocytic infiltrate in human breast cancers, the role of the major histocompatibility complex in cell-mediated immune mechanisms, and their associatin with prognostic indicators[J]. Surgery, 2003, 134(5): 827-34. |
| [21] | Leong PP, Mohammad R, Ibrahim N, et al. Phenotyping of lymphocytes expressing regulatory and effect or markers in infiltrating ductal carcinoma of the breast[J]. Immunol Lett, 2006, 102(2): 229-36. |
| [22] | Clemente CG, Mihm MC Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma[J]. Cancer, 1996, 77(7): 1303-10. |
| [23] | Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer[J]. Cancer Res, 1998, 58(16): 3491-4. |
| [24] | Ridolfi RL, Rosen PP, Port A, et al. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up[J]. Cancer, 1977, 40(4): 1365-85. |
| [25] | Kuroda H, Tamaru J, Sakamoto G, et al. Immunophenotype of lymphocytic infiltration in medullary carcinoma of the breast[J].Virchows Arch, 2005, 446(1): 10-4. |
| [26] | Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes[J]. Clin Cancer Res, 2008, 14(16): 5158-65. |
| [27] | Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial[J]. J Clin Oncol, 2009, 27(8): 1168-76. |
| [28] | Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes[J]. Clin Cancer Res, 2007, (8): 2329-34. |
| [29] | Parshad R, Kapoor S, Gupta SD, et al. Does famotidine enhance tumor-infiltrating lymphocytes in breast cancer?[J]. Acta Oncol, 2002, 41(4): 362-5. |
 2015, Vol. 42
2015, Vol. 42
