文章信息
- 杜敏娟, 徐晓光, 刘瑞琦, 杨亚琴, 蒋颖超, 吴丽丽.
- DU Minjuan, XU Xiaoguang, LIU Ruiqi, YANG Yaqin, JIANG Yingchao, WU Lili.
- 扁柏醇联合氯喹对人肺腺癌细胞凋亡的影响
- Effect of Hinokitiol Combined with Chloroquine on Apoptosis of Human Lung Adenocarcinoma Cells
- 肿瘤防治研究, 2015, 42(10): 970-973
- Cancer Research on Prevention and Treatment, 2015, 42(10): 970-973
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2015.10.004
-
文章历史
- 收稿日期: 2014-10-30
- 修回日期: 2014-03-25
2. 132021 吉林,吉化集团公司总医院 北华大学第二附属医院呼吸内科
2. Department of Respiratory, The General Hospital of CNPC in Jilin, The Second Affiliated Hospital of Beihua University, Jilin 132021, China
肺癌特别是肺腺癌,是世界上导致癌症患者死亡的主要原因之一[1]。传统的手术、放疗和化疗是治疗肺腺癌的主要方式,但往往复发率高。越来越多的证据显示,草药化合物已经成为治疗癌症的重要资源;从植物中提取的精油成分被用来治疗许多疾病,包括癌症[2, 3, 4]。从青森扁柏中提取的天然扁柏醇是托酚酮的一族,托酚酮具有优良的抗菌作用[5];而天然扁柏醇对EGFR-TKI-耐受的肺癌细胞系H1975有显著的治疗效果[6]。本研究旨在观察扁柏醇作用于人肺腺癌细胞H1975后细胞存活率的变化,以及联合氯喹和扁柏醇对H1975细胞凋亡的影响。
1 材料和方法 1.1 细胞培养和药物处理人肺腺癌细胞株H1975 CRL-5908™购于美国ATCC细胞库,用含10%胎牛血清,双抗(100u/ml青霉素、100 μg/ml链霉素)的RPMI1640培养液,在37℃、5%CO2饱和湿度的培养箱中培养,用0.4%胰蛋白酶消化传代培养,隔天传代。用含有不同浓度的扁柏醇和(或)联合20 μmol/L氯喹培养液处理H1975细胞24 h。
1.2 MTT法检测细胞存活率取生长状态良好的对数生长期H1975细胞,0.4%胰蛋白酶消化后,800 r/min离心5 min后制成细胞悬液,以每孔1×104个细胞的100 μl细胞悬液,接种于96孔板,培养24 h后,弃去原培养液,加入含2.5、5、10 μmol/L扁柏醇的10%胎牛血清RPMI 1640培养液,6个重复孔,未给予扁柏醇为对照组;联合应用组选择5 μmol/L扁柏醇与10 μmol/L氯喹联合应用。在培养箱中培养24 h,每孔加入10 μl MTT(10 μg/ml),将细胞移入培养箱继续培养4~6 h,可观察到紫色结晶颗粒,弃掉培养液,每个孔内加入150 μl DMSO,振荡5 min,用酶标仪490 nm波长条件下记录吸光度值。
1.3 Western blot检测凋亡相关蛋白药物处理24 h后,分别收集对照组和药物处理组细胞后,用预冷的PBS洗涤2次,将细胞转移至1.5 ml EP管中,加入含有蛋白酶抑制剂的120μl RIPA裂解液,超声5 s后,冰上孵育45 min,4℃ 4 500 r/min离心15 min,取上清液,采用BCA分析试剂盒测定蛋白浓度进行蛋白定量。上样前,煮沸10 min,37℃孵育10 min,30 μg蛋白质进行SDS-PAGF电泳分离,100 V转膜2 h,0.5%(W/V)脱脂牛奶室温封闭90 min,一抗4℃孵育过夜,PBST洗涤PVDF膜3次,时间分别为15、5、5 min,加入二抗室温孵育1 h,同上洗涤3次,根据ECL法显色,以β-actin蛋白为内参,采用凝胶图像分析软件测定目的条带灰度值。
1.4 统计学方法实验数据采用均值±标准差表示,实验至少重复3次,对实验数据采用SPSS13.0统计软件进行统计分析,P<0.05为差异具有统计学意义。
2 结果 2.1 扁柏醇对H1975细胞存活率的影响药物处理24 h后,与对照组相比较,扁柏醇2.5、5、10 μmol/L组逐渐出现严重的细胞皱缩和脱壁现象;与空白对照组相比,扁柏醇2.5、5、10μmol/L组细胞存活率逐渐降低,差异有统计学意义(P=0.036、0.028、0.011),见图 1。
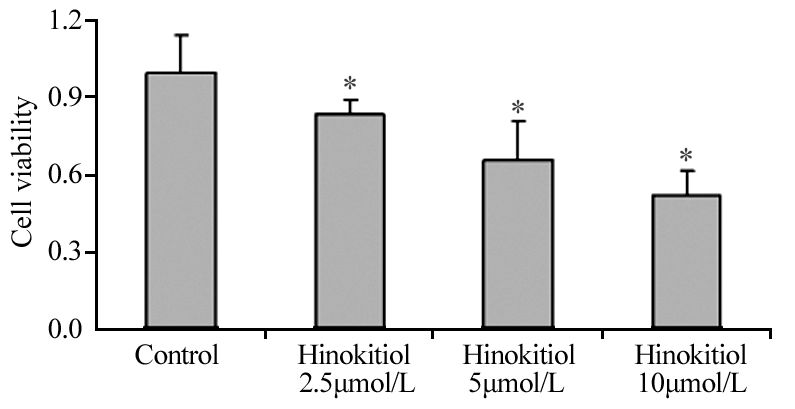
|
| *: P<0.05, compared with control group 图 1 不同浓度扁柏醇对H1975细胞存活率的影响 Figure 1 Effect of different concentrations of Hinokitiol on survival rate of H1975 cells |
药物处理24 h后,扁柏醇2.5、5、10 μmol/L组内凋亡相关蛋白Cleaved PARP、Cleaved Caspase-3、Bax的表达水平明显升高,见图 2,图 3,图 4,与对照组相比差异具有统计学意义(Cleaved PARP:P=0.024、0.030、0.006;Cleaved Caspase-3:P=0.038、0.033、0.021;Bax: P=0.018、0.022、0.034),提示扁柏醇能引起H1975细胞发生凋亡。
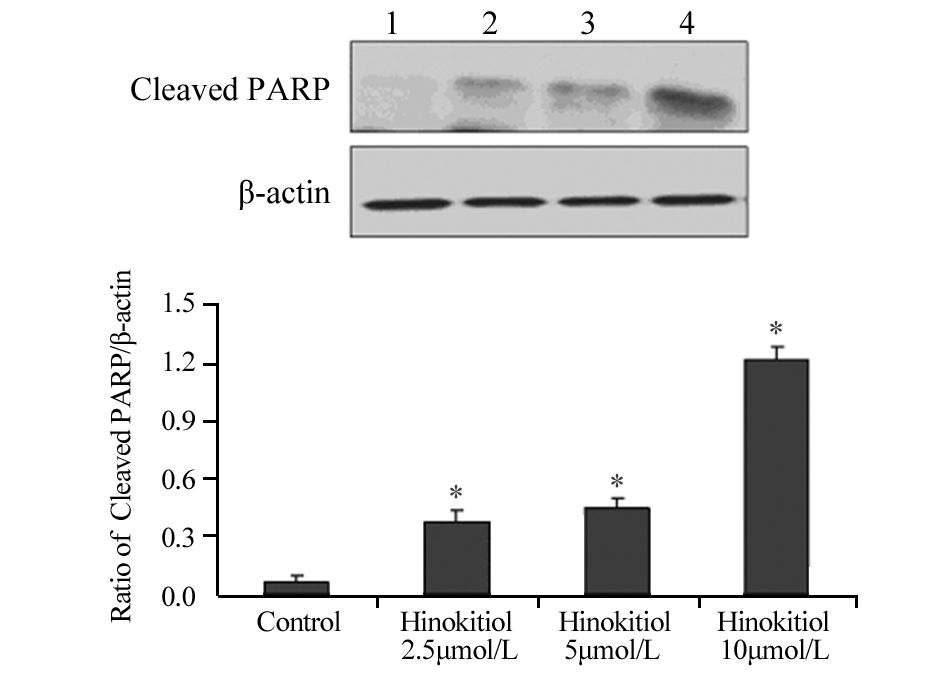
|
| 1: Control; 2: Hinokitiol 2.5μmol/L; 3: Hinokitiol 5μmol/L; 4: Hinokitiol 10μmol/L; *: P<0.05, compared with control group 图 2 不同浓度扁柏醇对H1975细胞凋亡相关蛋白Cleaved PARP表达水平的影响 Figure 2 Effect of different concentrations of Hinokitiol on expression level of apoptosis-related protein Cleaved PARP in H1975 cells |
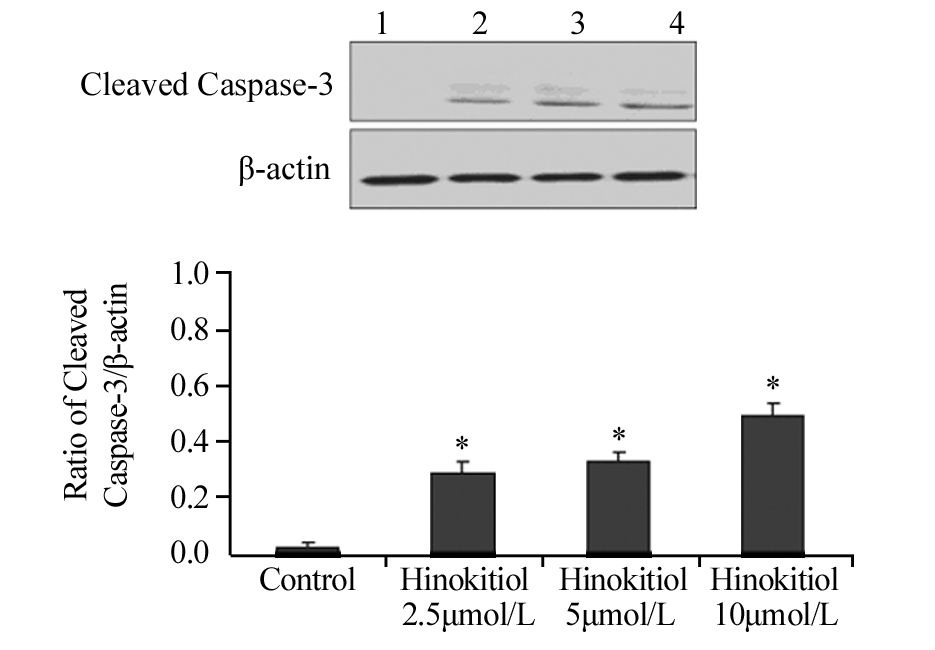
|
| 1: Control; 2: Hinokitiol 2.5μmol/L; 3: Hinokitiol 5μmol/L; 4: Hinokitiol 10μmol/L; *: P<0.05, compared with control group 图 3 不同浓度扁柏醇对H1975细胞凋亡相关蛋白Cleaved Caspase-3表达水平的影响 Figure 3 Effect of different concentrations of Hinokitiol on expression level of apoptosis-related protein Cleaved Caspase-3 in H1975 cells |
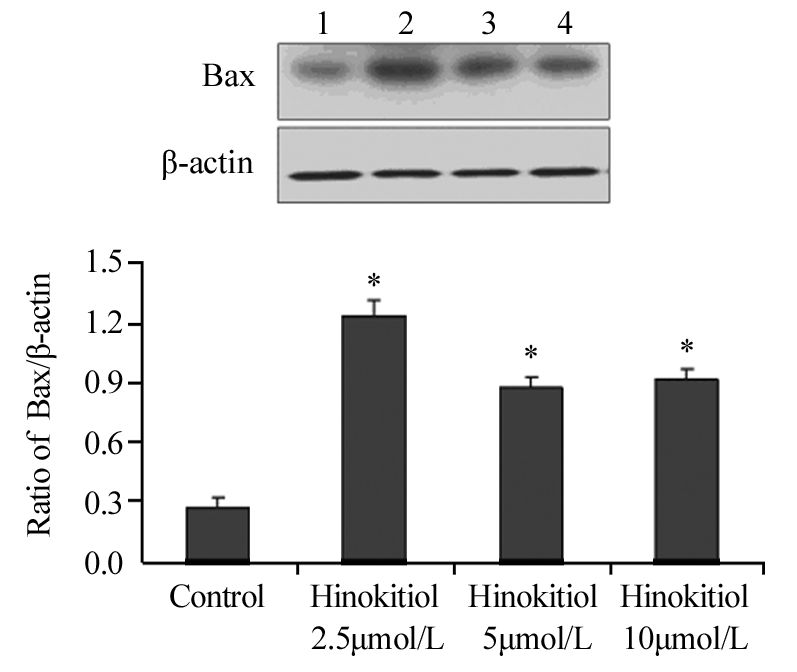
|
| 1: Control; 2: Hinokitiol 2.5μmol/L; 3: Hinokitiol 5μmol/L; 4: Hinokitiol 10μmol/L; *: P<0.05, compared with control group 图 4 不同浓度扁柏醇对H1975细胞凋亡相关蛋白Bax表达水平影响 Figure 4 Effect of different concentrations of Hinokitiol on expression level of apoptosis-related protein Bax in H1975 cells |
药物处理H1975细胞24 h后,与对照组相比,单加20 μmol/L氯喹组细胞存活率无明显差异;5μmol/L扁柏醇组和两者联合组的细胞存活率均明显降低,且两者联合组的细胞存活率明显低于单加扁柏醇组,差异均有统计学意义(P=0.043、0.048、0.042)见图 5,提示氯喹促进了扁柏醇对H1975细胞存活率的影响。
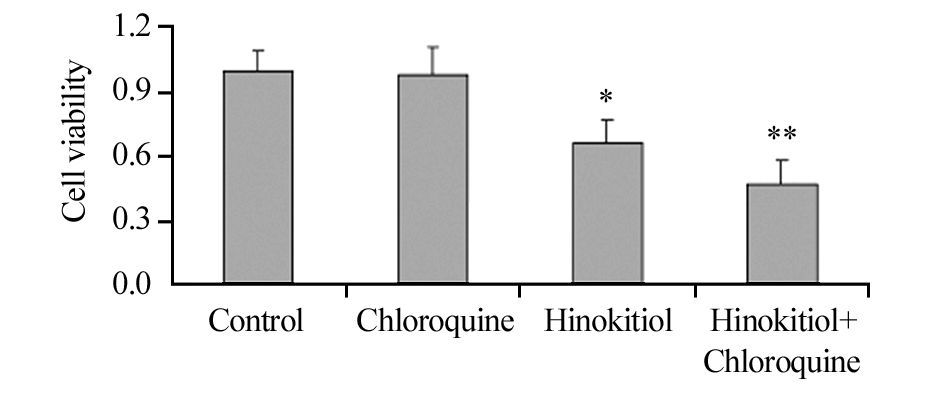
|
| *: P<0.05, compared with control group; **: P < 0.05, compared with Hinokitiol group and control group 图 5 氯喹联合扁柏醇对H1975细胞存活率的影响 Figure 5 Effect of Chloroquine combined with Hinokitiol on survival rate of H1975 cells |
药物处理H1975细胞24 h后,与对照组相比,单加20 μmol/L氯喹组凋亡相关蛋白Cleaved PARP和Cleaved Caspase-3的表达水平无明显差异;与单加5 μmol/L扁柏醇相比,联合组Cleaved PARP和Cleaved Caspase-3的表达水平明显增加,差异均有统计学意义(P=0.041、0.036),见图 6~图 7,提示联合氯喹可以明显增加扁柏醇对肿瘤的杀伤作用。
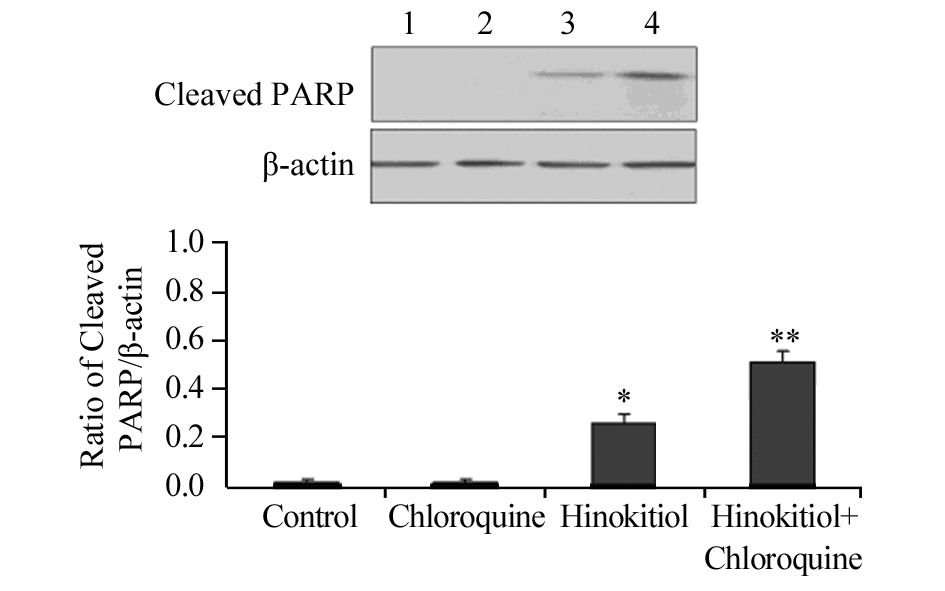
|
| 1: Control; 2: Chloroquine; 3: Hinokitiol; 4: Hinokitiol+Chloroquine; *: P<0.05, compared with control group;**: P<0.05, compared with Hinokitiol group and control group 图 6 氯喹联合扁柏醇对H1975细胞凋亡相关蛋白Cleaved PARP表达水平的影响 Figure 6 Effect of Chloroquine combined with Hinokitiol on expression level of apoptosis-related protein Cleaved PARP in H1975 cells |
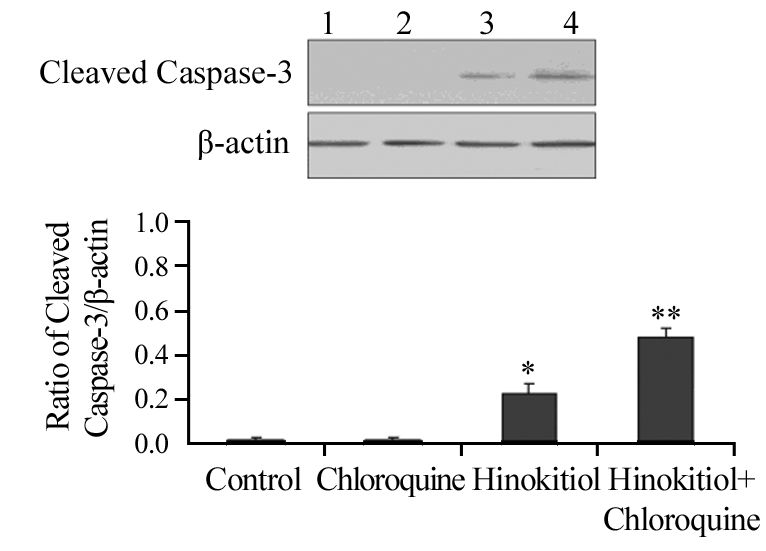
|
| 1: Control; 2: Chloroquine; 3: Hinokitiol; 4: Hinokitiol+Chloroquine; *: P<0.05, compared with control group;**: P<0.05, compared with Hinokitiol group and control group 图 7 氯喹联合扁柏醇对H1975细胞凋亡相关蛋白Cleaved Caspase-3表达水平的影响 Figure 7 Effect of Chloroquine combined with Hinokitiol on expression level of apoptosis-related protein Cleaved Caspase-3 in H1975 cells group |
肺癌治疗的主要手段包括手术、放疗、化疗、靶向药物治疗,不同分期采取不同的综合治疗策略。研究表明,扁柏醇具有抗炎症[7]、抗细菌[8]、抗真菌[9]、抗病毒的活性而扁柏醇对肺腺癌细胞影响及其作用机制仍不是很清楚。研究证明扁柏醇具有抑制黑色素瘤[10]、前列腺癌[11]、口腔癌[12]和结肠癌细胞[13]的作用。抗癌药物具有诱导癌细胞DNA链断裂,而影响细胞修复能力、诱导细胞周期停滞,或启动细胞程序性死亡[14, 15]。扁柏醇能诱导细胞周期停滞在S期,并导致细胞DNA损伤[6],因此扁柏醇可能会诱导癌症细胞凋亡。本研究中,我们发现扁柏醇呈剂量依赖性方式影响肺腺癌细胞H1975存活率,同时检测到凋亡相关蛋白(Cleaved PARP、Cleaved Caspase-3、Bax)表达升高。
氯喹具有弱化溶酶体内酸性环境,是一种自噬抑制剂,它能促进多种化疗药物对肿瘤细胞的杀伤作用[16, 17]。本研究显示,与单加扁柏醇相比,氯喹和扁柏醇联合应用明显地抑制肺腺癌H1975细胞存活率,细胞凋亡相关蛋白表达显著增加。这些结果说明,扁柏醇引起的肺腺癌H1975细胞中DNA损伤,从而抑制细胞增殖,氯喹通过抑制自噬活性促进扁柏醇诱导细胞凋亡的能力。同时本实验结果提示,氯喹抑制自噬后可能通过失活Bcl-2蛋白来诱导肺腺癌H1975启动程序性的细胞死亡,引起Caspase蛋白活化,而引起H1975细胞凋亡,进而促进扁柏醇诱导肺腺癌H1975细胞的凋亡。
总之,扁柏醇作为治疗癌症潜在的新型化疗药物,通过诱导肺腺癌H1975中DNA损伤而引起细胞凋亡,加入自噬抑制剂氯喹增强扁柏醇对肺腺癌H1975的杀伤作用,提高肺腺癌H1975对扁柏醇的敏感度。因此,氯喹和扁柏醇的联合应用成为治疗肺癌的潜在新策略,在体内作用和临床疗效值得进一步研究。
| [1] | Liu F, Yu G, Wang G, et al. An NQO1-initiated and p53-ndependent apoptotic pathway determines the anti-tumor effect ofanshinoneⅡA against non-small cell lung cancer[J]. PLoS One,012, 7(7): e42138. |
| [2] | Rogerio AP, Andrade EL, Leite DF, et al. Preventive andherapeutic anti-inflammatory properties of the sesquiterpenelpha-humulene in experimental airways allergic inflammation[J].r J Pharmacol, 2009, 158(4): 1074-87. |
| [3] | Darmanin S, Wismayer PS, Camilleri Podesta MT, et al. An extractrom Ricinus communis L. leaves possesses cytotoxic propertiesnd induces apoptosis in SK-MEL-28 human melanoma cells[J].at Prod Res, 2009, 23(6): 561-71. |
| [4] | Bhalla Y, Gupta VK, Jaitak V. Anticancer activity of essential oils: review[J]. J Sci Food Agric, 2013, 93(15): 3643-53. |
| [5] | Toshihiro OKABE, Kouji SAITO. Antibacterial and preservativeffects of natural Hinokitiol(β-Thujaplicin) extracted from wood[J]. Zhejiang Nong Ye Xue Bao, 1994, 6(4): 257-66 [树木中提取的天然扁柏醇的抗菌和保鲜效果[J]. 浙江农业学报, 1994, 6(4): 257-66.]. |
| [6] | Li LH, Wu P, Lee JY, et al. Hinokitiol induces DNA damagend autophagy followed by cell cycle arrest and senescence inefitinib-resistant lung adenocarcinoma cells[J]. PLoS One, 2014,(8): e104203. |
| [7] | Shih MF, Chen LY, Tsai PJ, et al. In vitro and in vivo therapeuticsf beta-thujaplicin on LPS-induced inflammation in macrophagesnd septic shock in mice[J]. Int J Immunopathol Pharmacol, 2012,5(1): 39-48. |
| [8] | Morita Y, Matsumura E, Okabe T, et al. Biological activity oflpha-thujaplicin, the isomer of hinokitiol[J]. Biol Pharm Bull,004, 27(6): 899-902. |
| [9] | Komaki N, Watanabe T, Ogasawara A, et al. Antifungal mechanismf hinokitiol against Candida albicans[J]. Biol Pharm Bull, 2008,1(4): 735-7. |
| [10] | Budihas SR, Gorshkova I, Gaidamakov S, et al. Selectivenhibition of HIV-1 reverse transcriptase-associated ribonuclease activity by hydroxylated tropolones[J]. Nucleic Acids Res,005, 33(4): 1249-56. |
| [11] | Liu S, Yamauchi H. Hinokitiol, a metal chelator derived fromatural plants, suppresses cell growth and disrupts androgeneceptor signaling in prostate carcinoma cell lines[J]. Biochemiophys Res Commun, 2006, 351(1): 26-32. |
| [12] | Shih YH, Chang KW, Hsia SM, et al. In vitro antimicrobial andnticancer potential of hinokitiol against oral pathogens and oralancer cell lines[J]. Microbiol Res, 2013, 168(5): 254-62. |
| [13] | Lee YS, Choi KM, Kim W, et al. Hinokitiol inhibits cell growthhrough induction of S-phase arrest and apoptosis in human colonancer cells and suppresses tumor growth in a mouse xenograftxperiment[J]. J Nat Prod, 2013, 76(12): 2195-202. |
| [14] | Calderón-Monta?o JM, Burgos-Morón E, Orta ML, et al.uanidine-reactive agent phenylglyoxal induces DNA damagend cancer cell death[J]. Pharmacol Rep, 2012, 64(6): 1515-25. |
| [15] | Hisatomi T, Sueoka-Aragane N, Sato A, et al. NK314 potentiatesntitumor activity with adult T-cell leukemia-lymphoma cells bynhibition of dual targets on topoisomerase Ⅱ{alpha} and DNAdependentrotein kinase[J]. Blood, 2011, 117(13): 3575-84. |
| [16] | Levy JM, Thorburn A.Targeting autophagy during cancer therapyo improve clinical outcomes[J]. Pharmacol Ther, 2011, 131(1):30-41. |
| [17] | Kimura T, Takabatake Y, Takahashi A, et al. Chloroquine in cancerherapy: a double-edged sword of autophagy[J]. Cancer Res,013, 73(1): 3-7. |
 2015, Vol. 42
2015, Vol. 42
