2.输血科
2.Department of Blood Transfusion
在世界范围内,胆管癌占所有胃肠道肿瘤的 3%,是第二常见的肝胆肿瘤,也是我国常见的恶 性肿瘤之一。根据部位将胆管癌分为肝内和肝外 胆管癌。手术切除是肝外胆管癌可能治愈的唯一手 段,但大多数患者发现时均为晚期,即使能手术的 患者也有很高的复发率。总体上肝外胆管癌的预后 不良,其预后涉及到病理学因素包括:肿瘤分化程 度、肿瘤分期、淋巴结有无转移、神经有无侵犯、 脉管有无癌栓、肿瘤浸润深度和范围、肿瘤大小、 手术切缘等,这些均可能是影响确定治疗方案的重 要因素,因此本研究通过总结肝外胆管癌患者病理 特点及随访,旨在对影响肝外胆管癌生存率的病理 因素进行研究,并探讨各因素之间的关系。 1 资料和方法 1.1 一般资料
收集2000年1月—2008年4月我院手术切除,均 经病理学证实为肝外胆管癌,并且资料完整的患 者124例。男88例,女36例,发病年龄32~82岁,中 位年龄60岁。我国胆管癌的发病年龄分布在20~89 岁,平均59岁,发病高峰年龄为50~60岁。胆管癌男 性多于女性,男与女性发病比率为(1.5~3.0):1, 但原因尚不明确。按分化程度区分,高分化15例, 中分化74例,低分化35例,见表 1。
|
|
表 1 124例肝外胆管癌患者临床病理特点 Table 1 Clinicopathological features of 124 patients with cholangiocarcinoma |
所有数据均采用SPSS 19.0软件进行统计学分 析,单因素分析用Log rank检验,以Cox回归进行多 因素生存分析。以P<0.05为差异有统计学意义。 2 结果 2.1 总生存期
随访截止时间为2013年4月30日,全部124例患 者,1、3和5年生存率分别为77.4%、40.3%和16.9%。 2.2 单因素分析
各观察指标与肝外胆管癌术后预后的关系, 见表 2。经Log rank单因素分析显示,其中有4个因 素P<0.05,即肿瘤局部浸润、淋巴结转移、分化 程度、切缘癌细胞浸润,再将以上所有指标进入 Cox模型进行多因素回归分析。
|
|
表 2 124例肝外胆管癌患者各临床病理特征的单因素分析 Table 2 Univariate survival analysis of clinicopathological features of 124 patients with cholangiocarcinoma |
按α=0.05的标准,将各变量通过Cox多因素分 析,得出和预后有关的重要独立因素,结果见表 3。各独立预后因素的K-M曲线,见图 1~4。
|
|
表 3 124例肝外胆管癌患者Cox比例风险模型多因素回归分析 Table 3 Multivariate regression analysis of factors for 124 patients with cholangiocarcinoma by Cox proportional hazard model |
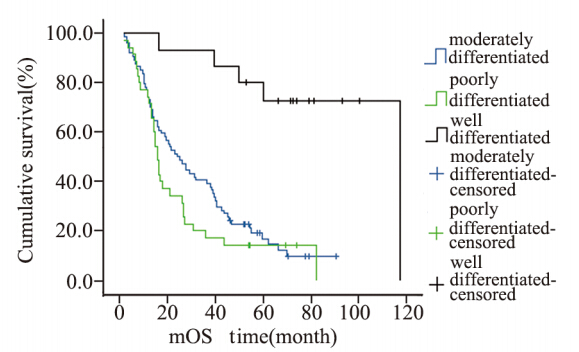 |
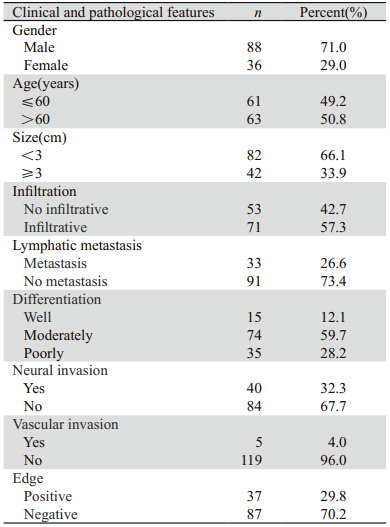
图1 124例肝外胆管癌患者肿瘤分化程度生存曲线 Figure 1 Cumulative survival curves of tumor differentiation degree in 124 patients with cholangiocarcinoma |
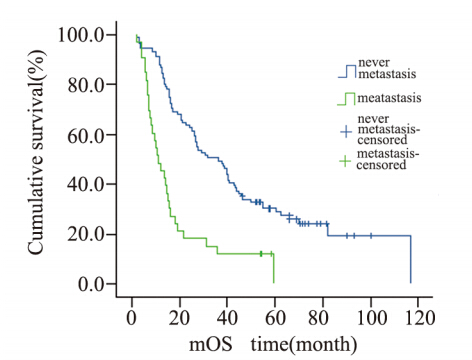 |
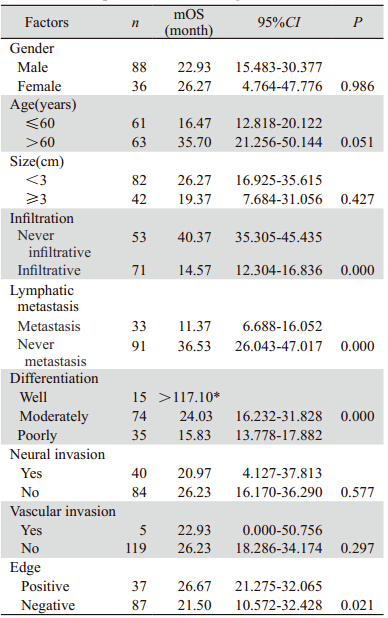
图2 124例肝外胆管癌患者淋巴结转移情况生存曲线 Figure 2 Cumulative survival curves of lymphatic metastasis in 124 patients with cholangiocarcinoma |
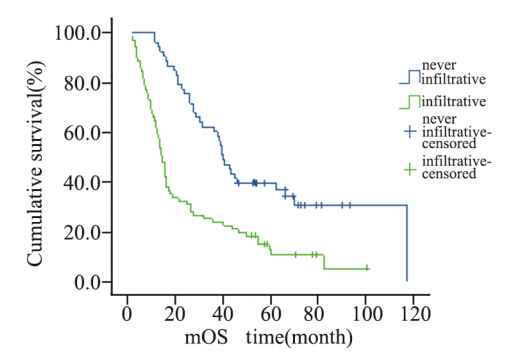 |
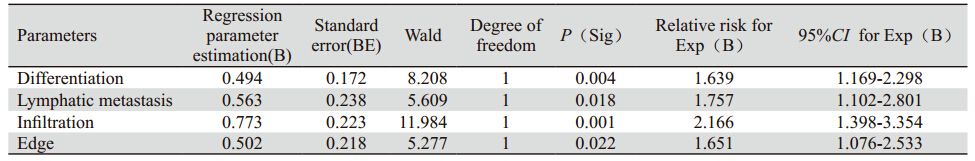
图3 124例肝外胆管癌患者周围浸润生存曲线 Figure 3 Cumulative survival curves of infiltration in 124 patients with cholangiocarcinoma |
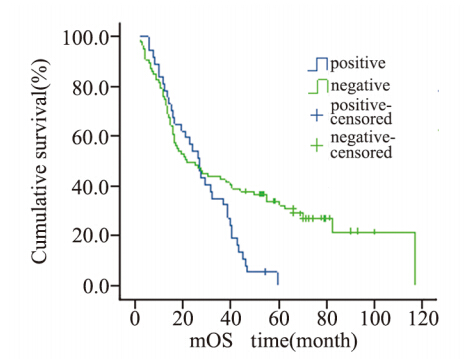 |
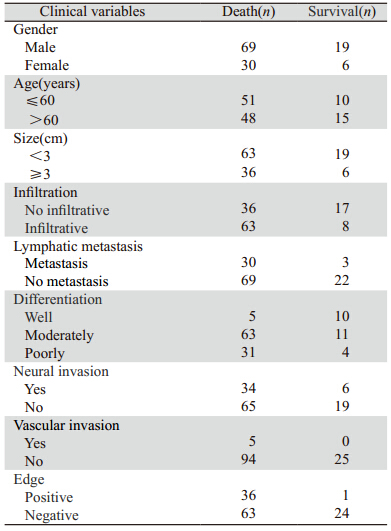
图4 124例肝外胆管癌患者切缘情况生存曲线 Figure 4 Cumulative survival curves of incisal edge in 124 patients with cholangiocarcinoma |
肝外胆管癌患者的病理因素与生存的关系可以 看出一些病理结果直接影响患者的生存,见表 4。
|
|
表 4 124例肝外胆管癌患者Cox比例风险模型多因素回归分析 Table 4 The relationship of clinical variables with survival of 124 patients with cholangiocarcinoma |
近年来肝外胆管癌的发病率呈逐年上升趋 势,晚期肝外胆管癌的预后很差,中位生存期不 足1年[1],手术切除是肝外胆管癌可能治愈的唯一 手段[2],对于晚期无法行手术切除的病例,能否通 过化疗使患者达到治疗肿瘤的目的,化疗是否也 是影响预后的因素之一?晚期胆管癌迄今缺乏公 认的化疗方案,仅有一些国内外的临床试验进行 了相应的研究[3,4,5,6,7],但效果不尽相同,本研究入选 的病例大部分已行根治性手术,术后化疗的病例较少,且化疗方案也不一致,若将化疗是否影响 预后作为一个因素进行分析,病例数不均衡,达 不到统计学上的要求,其结果不一定可信,故本 研究没有将是否化疗作为影响因素进行统计学分 析,而是将可能对肝外胆管癌预后有影响的病理 因素进行单因素和多因素分析,明确影响肝外胆 管癌预后在病理方面的危险因素及各危险因素之 间的交互作用。本研究显示,肿瘤分化程度、淋 巴结转移情况、肿瘤周围浸润情况、切缘癌细胞 浸润残存情况为影响肝外胆管癌患者长期生存的 独立预后因素。肿瘤分化程度较低、淋巴结癌转 移、肿瘤周围浸润、切缘阳性为死亡危险因素。另 外,1、3及5年生存率高于相关文献报道[8,9,10,11],考虑 可能与本组患者肿瘤分期较早且大部分患者行根 治手术有关。
肿瘤分化程度可显著影响胆管癌患者的预后 [12,13,14] 。低分化癌的预后比高分化癌的预后差,这主要与 低分化癌浸润及转移的特征明显、容易发生早期转移有关,这与临床远期疗效的观察结果相符。 本研究也显示了同样的结果,因此尽早明确肿瘤 的分化程度,可以为临床提供制定出最佳治疗方 案的依据,可以通过术前活检病理诊断和术中冰冻病理诊断明确肿瘤的分化程度。
肿瘤的淋巴结转移是影响预后的独立因素[15], 胆管癌早期即可沿胆管周围淋巴管、血管、神经 周围间隙及疏松纤维结缔组织等发生多途径浸润转移,淋巴结发生癌转移说明肿瘤已经进入脉管 系统进行播散,国外文献报道[16],在发生淋巴结 转移的病例中,多于2个淋巴结发生癌转移其预 后不良。本研究仅显示了发生淋巴结癌转移的病 例比淋巴结未发生癌转移的病例预后差,并没有 对发生癌转移的淋巴结数目进行统计学分析。因 此,术中应尽可能的清扫区域淋巴结,对于判断 预后有一定的意义。
肝外胆管癌向周围组织的浸润生长被认为是 影响肝外胆管癌预后的主要因素[11,17,18,19,20,21]。因为早期 胆管癌尚未发生远处转移,而主要以沿着胆管向 上或向下方式呈浸润性生长,肿瘤一旦发生向周 围浸润性生长,提示肿瘤细胞生长活跃。因此, 要求手术切除范围更大,且不易达到根治性切除 的目的。
切缘有癌细胞浸润的提示预后差[13,22,23],切缘 阳性即未达到R0切除,体内仍然部分残存活的肿 瘤细胞,这是导致肿瘤复发的重要原因。通过本研 究同样发现,切缘阳性的病例发生复发转移的总 生存较切缘阴性的病例时间短。国外研究表明[24], 胆管癌细胞沿黏膜下浸润生长长度通常<10 mm, 所以,手术切除切缘距肿瘤10 mm以上,通常可以 获得切缘无癌细胞浸润,但是还需要术中冰冻病 理诊断证实切缘情况,以期达到R0切除,尽可能 延长患者的总生存期。
本研究样本量不大,故下一步将进行大样本 量的统计并且完善国内外文献中涉及而本研究未 完成的统计,进一步证实影响肝外胆管癌预后的 病理因素。
| [1] | Hezel AF,Zhu AX. Systemic therapy for biliary tract cancers[J]. Oncologist,2008,13(4):415-23. |
| [2] | Wu MC.Huang Jiasi Surgery[M].7th Edition.Beijing:People’s Medical Publishing House,2008:1822-5.[吴孟超.黄家驷外科学[M].7版.北京:人民卫生出版社,2008:1822-5.] |
| [3] | Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials [J]. Br J Cancer,2007, 96(6):896-902. |
| [4] | Kornek GV, Schuell B, Laengle F, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer:A randomised phase II trial[J]. Ann Oncol,2004,15(3):478-83. |
| [5] | Ducreux M, Van Cutsem E, Van Laethem JL, et al. A randomised phase Ⅱtrial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial[J]. Eur J Cancer,2005, 41(3):398-403. |
| [6] | Rao S, Cunningham D, Hawkins RE, et al. Phase Ⅲstudy of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer[J]. Br J Cancer,2005,92(9):1650-4. |
| [7] | Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase Ⅱstudy[J].J Clin Oncol, 2001, 19(20): 4089-91. |
| [8] | Hong SM, Cho H, Moskaluk CA, et al. Measurement of the invasion depth of extrahepatie bile duct carcinoma: an alternative method overcoming the current T classification problems of the AJCC staging system[J]. Am J Surg Patho1,2007,31(2):199-206. |
| [9] | Nelson JW, Ghafoori AP, Willett CG, et al.Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma[J]. Int J Radiat Oncol Biol Phys,2009,73(1):148-53. |
| [10] | Woo SM,Ryu JK,Lee SH,et al. Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma [J]. Ann Surg Oncol, 2007,14(11):3195-201. |
| [11] | Woo SM,Ryu JK,Lee SH,et al. Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma [J]. Ann Surg Oncol, 2007,14(11):3195-201. |
| [12] | Ebata T, Nagino M, Kamiya J, et al. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases[J]. Ann Surg,2003,238(5):720-7. |
| [13] | Rea DJ, Munoz-Juarez M, rnell MB, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients[J]. Arch Surg,2004,139(5):514-23. |
| [14] | Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document [J]. Gut,2002,51 Suppl 6:VI1-9. |
| [15] | Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection[J]. World J Surg,2009,33(6):1247-54. |
| [16] | Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma[J]. World J Surg,2005,29(6):728-33. |
| [17] | d e J o n g MC , H o n g S M, A u g u s t i n e M M,e t a l. H i l a r cholangiocarcinoma: tumor depth as a predictor of outcome[J]. Arch Surg,2011,146(6):697-703. |
| [18] | Vern-Gross TZ, Shivnani AT, Chen K, et al. Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis[J]. Int J Radiat Oncol Biol Phys,2011,81(1): 189-98. |
| [19] | Nagahashi m, Shirai Y, Wakai T, et al. Depth of invasion determines the postresectional prognosis for patients with T1 extrahepatic cholangiocarcinoma[J]. Cancer,2010,116(2):400-5. |
| [20] | Hong SM, Cho H, Moskaluk CA, et al. Measurement of the invasion depth of extrahepatic bile duct carcinoma: An alternative method overcoming the current T classification problems of the AJCC staging system[J].Am J Surg Patho1,2007,31(2):199-206. |
| [21] | Kloek JJ, Ten Kate FJ, Busch OR, et al. Surgery for extrahepatic cholangiocarcinoma: predictors of survival[J]. HPB(Oxford), 2008,10(3):190-5. |
| [22] | Ribero D, Amisano M, Lo Tesoriere R, et al. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma[J]. Ann Surg,2011,254(5):776-81;discussion 781-3. |
| [23] | Tamandl D, Herberger B, Gruenberger B, et al. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma[J]. Ann Surg Oncol,2008,15(10):2787-94. |
| [24] | Sakamoto E,Nimura Y,Hayakawa N,et al.The pattern of infiltration at the proximal border of hilar bile duct carcinoma:a histologic analysis of 62 resected cases[J].Ann Surg,1998,227(3):405-11. |
 2014, Vol.41
2014, Vol.41