0 引言
头颈部肿瘤以鳞癌为其主要的病理类型。头 颈部肿瘤患者的管理主要集中于经验丰富的多学 科团队及相关组织、机构。基于大量的原始研究 证据,一些机构发布了自己的临床指南。但是, 并不是所有的指南都是高质量的。本文采用临床 指南研究与评价系统( Appraisal of Guideline for REsearch and Evaluation,AGREE) 评价国内外头 颈部鳞癌相关指南的质量,为我国头颈部鳞癌临 床指南的编制工作提供借鉴。 1 资料和方法 1.1 研究资料
计算机检索PubMed、Embase、U.S. National Guideline Clearing-house, the Canadian Medical Association Infobase, the Guidelines International Network, the Scottish Intercollegiate Guidelines Network, CMAdisc数据库文献资料,截止2012年10月。
英文检索词:head and neck cancer;squamous cell of head and neck; guidelines。中文检索词:头 颈部肿瘤;指南;规范。 1.2 纳入与排除标准
纳入公开发布的头颈部鳞癌指南,指南针对 的主题、研究方法和分期不限,语种仅为中文、 英文。排除关于临床指南的介绍、评析、应用指 导、应用效果评价、勘误表和不同语种版本及重 复收录的指南。 1.3 评价与分析方法
纳入的指南采用AGREE Ⅱ[1]工具评价其质 量。评价前培训评价者并分析评价结果的一致 性,一致性达到可接受的最低水平后开始评价。 1.4 评价工具及方法
AGREE工具评价指南由6个领域组成:(1) 范围和目的;(2)制定指南的参与人员;(3) 制定的严谨性;(4)清晰性与可读性;(5)适 用性;(6)编撰的独立性。
每个条目根据评价标准按7级划分等级:从1 分(非常不同意)到7分(非常同意)进行评分。 每个领域最终得分由每个评分人员通过以下计算 方法得到:(实际得分最低可能得分)/(最高可 能得分最低可能得分)×100% 。 1.5 统计学方法
在开始进行评价前,随机抽取6篇文献,两 名评价员独立评价[2]。结果采用组内相关系数 (Interclass Correlation Coefficient, ICC)及其95%可 信区间(95%CI)描述评价标准的符合程度或一致 性[3]。一致性超过最低水平0.75后方可开始独立纳 入研究质量。各领域得分采用平均值±标准误(x±s) 表示。独立样本t检验用于比较两组的差异,单因 素方差分析用于比较多组间的差异,P<0.05为差 异有统计学意义。采用SPSS18.0软件进行统计分 析。
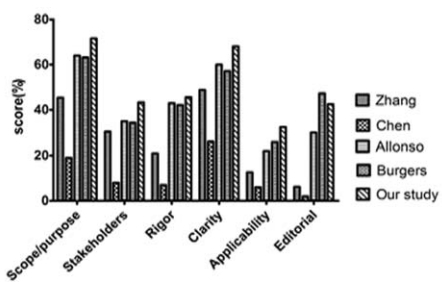 |
图 1本文与Chen、Alonso-Coello、Zhang、Burgers研究的指南质量的比较 Figure 1Comparison of the quality of the guidelines included in this report with those included by Chen, Alonso-Coello, Zhang and Burgers |
共检索出514篇相关文献,最终纳入49篇头颈 部鳞癌指南,见表 1。最早发布于2001年,最近发 布于2012年。指南覆盖15个国家和地区。39篇指 南由专业的指南机构、组织发布,10篇以个人名 义发表。29篇指南基于循证方法学制定,其余的 指南制定基于专家意见、临床经验、回顾性研究 等方法。指南关注的问题集中于放疗、化疗、手 术、肿瘤筛查等领域。22.4%的指南有过更新。
|
|
表 1 49部指南的基线资料 Table 1 The basic information of 49 guidelines |
正式评价前,两名评价者采用AGREE Ⅱ对随 机抽取的6篇指南进行独立评价,两位评价员的评 价结果的ICC为0.96(95%CI:0.93~0.98)。评价员对 评价条目的理解、判断及评价结果的一致性较高。 2.3 纳入指南的整体质量
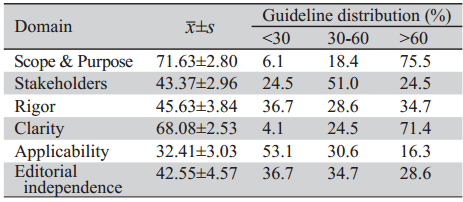
49篇指南的整体质量中等。六个领域得分分 别为:(71.63±2.80)%、(43.37±2.96)%、(45.63± 3.84)%、(68.08±2.53)%、(32.41±3.03)%、(42.55± 4.57)%,见表 2。
|
|
表 2 指南得分及各领域得分分布情况 Table 2 Guideline score (x±s) and Guideline distribution (%) according to scores in each domain assessed by the AGREE II instrument |
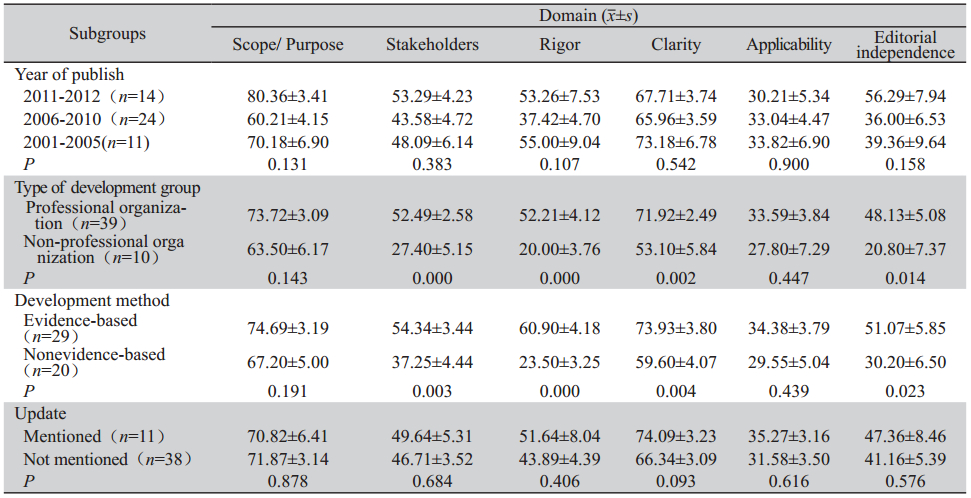
根据发表年限(2001-2005,2006-2010, >2010)、指南制定机构类型(专业机构与非专业 机构)、更新与否、制定的方法学(基于循证医 学、未基于循证医学)对纳入的指南的各领域得 分进行亚组分析,见表 3。
|
|
表 3 纳入指南的各领域亚组分析结果 Table 3 The subgroup analysis results of each domain of included guidelines |
本研究是首次采用专业的评价工具系统进行 的国内外头颈部鳞癌指南的质量评价,纳入指南 的整体质量中等。所纳入的指南各领域得分较好 的为“范围和目的”及“清晰性”。大多数的指南详细 描述了他们的特定目标人群和集中关注的临床问 题。近几年来,“范围和目的”这一领域的得分交 前有所上升。
“制定的严谨性”这一领域得分较低。可能存在 以下原因:首先,大部分的指南没有报道其系统的 检索、筛选证据的方法;其次,只有极少数的指 南在发布之间进行了外审工作;再者,极少数的 指南对证据进行了测试验证;最后,只有22.4%的 指南进行了更新或拟定了更新时间。
“参与人员”这一领域的得分也较低,这与缺乏 多学科指南制定小组有关。所纳入指南中,79.6% 由专业的指南制定小组、机构、组织制定,然 而,几乎没有指南在制定过程中考虑目标人群对 卫生服务的体验和期望,也没有让目标人群参与 指南制定小组。
得分最低的两个领域是“应用性”及“编撰的独 立性”。这可能与较差的表达有关,指南制定人员 应能提供用于实践的推荐建议和工具,例如:简 介、快速参考手册、教具等。几乎所有的医学文 献中都要求作者详细说明是否存在利益冲突,这 已成为共识,然而,在指南制定中,对于是否存 在利益冲突及赞助商等却未能引起足够的重视。
Chen等[4]对国内115部临床指南采用AGREE Ⅱ
进行评价,国内指南在“范围和目的”“清晰性”两个
领域得分较高,仅分别为19%、26%,而“参与人
员”、“制定的严谨性”及“应用性”“编撰的独立性”
的得分分别为8%、7%、6%、2%。Zhang等[5]对国
内21部耳鼻喉指南进行评价的结果较Chen等[4]的
稍好,六个领域的得分分别为:45.4%、30.4%、
20.9%、48.8%、12.6%、6.2%。然而,相较于国
际指南的平均值仍相差甚远。Alonso-Coello等[6]
对1980至2010年期间全球各领域指南进行评价,
六个领域得分分别为:64%、60%、35%、43%、
22%、30%。Burgers等[7]曾对100部指南进行系统
评价,其中包含32部肿瘤学指南,研究显示,肿
瘤学指南较非肿瘤学指南得分稍好,六个领域
得分分别为:62.5%、37.2%、48.8%、60.0%、
19.1%、52.8%。本研究结果与国际平均值较为
接近,然而,在所纳入的指南中,未有一部是中
国原创。国内对于头颈部肿瘤的诊疗基本依靠国
外的相关指南及相关权威人士编写的个人指南,
如:美国国立癌症综合信息网头颈部肿瘤(中国
版)、头颈肿瘤治疗专家共识等。在政府指导下
开展头颈部鳞癌防治是我国头颈部鳞癌防治的基本
策略。目前,卫生部已颁布我国肺癌、直肠癌等相
关诊治规范、指南,但未见头颈鳞癌的诊治规范。
| [1] | AGREE next steps consortium (2009). The AGREE II instrument (Electronic version), 2011. |
| [2] | Chen yi, Hu shilian, Li youping, et al. Guidelines concerning pharmacological intervention in simple hypertension: a systematic review[J].Zhongguo Xun Zheng Yi Xue Za Zhi, 2012, 12(10): 1180-94.[陈尹, 胡世莲, 李幼平,等.全球药物干预治疗单纯性高血压指南的系统评价[J].中国循证医学杂志,2012,12(10):1180-94.] |
| [3] | Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability[J]. Psychol Bull, 1979, 86(2): 420-8. |
| [4] | Chen YL,Yao L,Xiao XJ,et al.Quality assessment of clinical guidelines in China:1993-2010[J]. Chin Med J(Engl), 2012, 125(20): 3660-4. |
| [5] | Zhang ZW, Liu XW, Xu BC, et al.Analysis of quality of clinical practice guidelines for otorhinolaryngology in China[J]. PloS One, 2013,8(1): e53566. |
| [6] | Alonso-Coello P, Irfan A, Solà I, et al. The quality of clinical practice guidelines over the last two decades: a systematic review of guideline appraisal studies[J]. Qual Saf Health Care,2010, 19(6): e58. |
| [7] | Burgers JS, Fervers B, Haugh M, et al. International assessment of the quality of clinical practice guidelines in oncology using the Appraisal of Guidelines and Research and Evaluation Instrument[J]. J Clin Oncol, 2004, 22(10): 2000-7. |
| [8] | Expert Panel on Radiation Oncology-Head & Neck Cancer, Yeung AR, Garg MK, et al. ACR Appropriateness Criteria?ipsilateral radiation for squamous cell carcinoma of the tonsil[J]. Head Neck, 2012, 34(5): 613-6. |
| [9] | Rethman MP, Carpenter W, Cohen EE, et al. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas[J]. Tex Dent J, 2012, 129(5): 491-507. |
| [10] | Bernier J, Russi EG, Homey B, et al. Management of radiation dermatitis in patients receiving cetuximab and radiotherapy for locally advanced squamous cell carcinoma of the head and neck: proposals for a revised grading system and consensus management guidelines[J]. Ann Oncol, 2011, 22(10): 2191-200. |
| [11] | Mesía Nin R, Pastor Borgo?ón M, Cruz Hernández JJ, et al. SEOM clinical guidelines for the treatment of head and neck cancer[J]. Clin Transl Oncol, 2010, 12(11): 742-8. |
| [12] | Grégoire V, Lefebvre JL, Licitra L, et al. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2010, 21 Suppl 5: v184-6. |
| [13] | Quon H, Yom SS, Garg MK,et al. ACR Appropriateness Criteria: local-regional therapy for resectable oropharyngeal squamous cell carcinomas[J]. Curr Probl Cancer, 2010, 34(3): 175-92. |
| [14] | Ramos M,Benavente S,Giralt J. Management of squamous cell carcinoma of the head and neck: updated European treatment recommendations[J]. Expert Rev Anticancer Ther, 2010, 10(3): 339-44. |
| [15] | Wee JT, Anderson BO, Corry J,et al. Management of the neck after chemoradiotherapy for head and neck cancers in Asia: consensus statement from the Asian Oncology Summit 2009[J]. Lancet Oncol, 2009, 10(11): 1086-92. |
| [16] | Alkureishi LW, Burak Z, Alvarez JA,et al. Joint practice guidelines for radionuclide lymphoscintigraphy for sentinel node localization in oral/oropharyngeal squamous cell carcinoma[J]. Ann Surg Oncol, 2009, 16(11): 3190-210. |
| [17] | Porceddu SV, Sidhom M, Foote M, et al. Predicting regional control based on pretreatment nodal size in squamous cell carcinoma of the head and neck treated with chemoradiotherapy: a clinician’s guide[J]. J Med Imaging Radiat Oncol, 2008, 52(5): 491-6. |
| [18] | Chopra S, Gupta T, Agarwal JP, et al. Re-irradiation in the management of isolated neck recurrences: current status and recommendations[J]. Radiother Oncol, 2006, 81(1):1-8. |
| [19] | Bilde A, von Buchwald C, Johansen J, et al. The Danish national guidelines for treatment of oral squamous cell carcinoma[J]. Acta Oncol, 2006, 45(3): 294-9. |
| [20] | Grégoire V, Eisbruch A, Hamoir M, et al. Proposal for the delineation of the nodal CTV in the node-positive and the postoperative neck[J]. Radiother Oncol, 2006, 79(1): 15-20. |
| [21] | Hamoir M, Vander Poorten V, Chantrain G,et al. Initial work-up in head and neck squamous cell carcinoma[J]. B-ENT, 2005, 1: 129-32. |
| [22] | Mazeron JJ, Ardiet JM, Haie-Méder C, et al. GEC-ESTRO recommendations for brachytherapy for head and neck squamous cell carcinomas[J]. Radiother Oncol, 2009, 91(2): 150-6. |
| [23] | Brown J, Chatterjee R, Lowe D, et al. A new guide to mandibular resection for oral squamous cell carcinoma based on the Cawood and Howell classification of the mandible[J]. Int J Oral Maxillofac Surg, 2005, 34(8): 834-9. |
| [24] | Hodson DI, Browman GP, Thephamongkhol K, et al. The role of amifostine as a radioprotectant in the management of patients with squamous cell head and neck cancer[J]. Current Oncology, 2003, 10(3): 146-60. |
| [25] | Cancer Care Ontario. Hyperfractionated radiotherapy for locally advanced squamous cell carcinoma of the head and neck[OL].Toronto, Ontario: Cancer Care Ontario,2003. |
| [26] | Yoo J, Lacchetti C, Hammond A, et al. The role of endolaryngeal surgery (with or without laser) versus radiotherapy in the management of early (T1) glottic cancer[OL].Toronto, Ontario: Cancer Care Ontario,2012. |
| [27] | Expert Panel on Radiation Oncology-Head and Neck, Salama JK, Saba N, et al. ACR Appropriateness Criteria? adjuvant therapy for resected squamous cell carcinoma of the head and neck[J]. Oral Oncol, 2011,47(7):554-9. |
| [28] | Orphanidou C, Biggs K, Johnston ME, et al. Prophylactic feeding tubes for patients with locally advanced head-and-neck cancer undergoing combined chemotherapy and radiotherapy-systematic review and recommendations for clinical practice[J]. Curr Oncol, 2011, 18(4):e191-201. |
| [29] | O’Sullivan B, Rumble RB, Warde P. The role of IMRT in head and neck cancer[OL]. Toronto, Ontario: Cancer Care Ontario,2011. |
| [30] | McDonald MW, Lawson J, Garg MK, et al. ACR appropriateness criteria retreatment of recurrent head and neck cancer after prior definitive radiation expert panel on radiation oncology-head and neck cancer[J].Int J Radiat Oncol Biol Phys,2011, 80(5):1292-8. |
| [31] | Dutch Head and Neck Oncology Cooperative Group.Hypopharyngeal cancer[OL].Netherlands:Dutch Head and Neck Oncology Cooperative Group,2007. |
| [32] | Kaanders JH, Hordijk GJ, Dutch Cooperative Head and Neck Oncology Group. Carcinoma of the larynx: the Dutch national guideline for diagnostics, treatment, supportive care and rehabilitation[J]. Radiother Oncol,2002, 63(3):299-307. |
| [33] | GUIDELINE POFT. The management of head and neck cancer in ontario: organizational and clinical practice guideline recommendations[OL]. Toronto, Ontario: Cancer Care Ontario,2009. |
| [34] | Cripps C, Winquist E, Devries MC, et al. Epidermal growth factor receptor targeted therapy in stages III and IV head and neck cancer[J]. Curr Oncol, 2010, 17(3): 37-48. |
| [35] | Greenhalgh J,Bagust A,Boland A,et al.Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck[J].Health Technol Assess,2009,13 Suppl 3:49-54. |
| [36] | Cancer Care Ontario. PET imaging in head and neck cancer[OL].Toronto, Ontario: Cancer Care Ontario,2012. |
| [37] | Health Partners Dental Group.Health Partners Dental Group and Clinics oral cancer guideline[OL].Minneapolis, American: Health Partners Dental Group,2007. |
| [38] | Scottish Intercollegiate Guidelines Network. Diagnosis and management of head and neck cancer. A national clinical guideline[internet]. Edinburgh, Scotland:Scottish Intercollegiate Guidelines Network,2006. |
| [39] | American Society of Clinical Oncology, Pfister DG, Laurie SA,et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer[J].J Clin Oncol,2006, 24(22):3693-704. |
| [40] | National Institute for Clinical Excellence. Improving outcomes in head and neck cancers (CSGHN) [OL].London: National Institute for Clinical Excellence,2004. |
| [41] | British Association of Head and Neck Oncologists. Practice care guidance for clinicians participating in the management of head and neck cancer patients in the UK. Drawn up by a Consensus Group of Practising Clinicians[J]. Eur J Surg Oncol, 2001, 27 Suppl A:S1-17. |
| [42] | Manikantan K, Khode S, Dwivedi RC, et al. Making sense of posttreatment surveillance in head and neck cancer: when and what of follow-up[J]. Cancer Treat Rev, 2009, 35(8):744-53. |
| [43] | Cancer Care Ontario. The role of post-operative chemoradiotherapy for squamous cell carcinoma of the head and neck[OL].Toronto, Ontario: Cancer Care Ontario,2004. |
| [44] | Cancer Care Ontario. Symptomatic treatment of radiation-induced xerostomia in head and neck cancer patients[OL].Toronto, Ontario: Cancer Care Ontario,2004. |
| [45] | Tolentino Ede S, Centurion BS, Ferreira LH, et al. Oral adverse effects of head and neck radiotherapy: literature review and suggestion of a clinical oral care guideline for irradiated patients[J]. J Appl Oral Sci,2011, 19(5):448-54. |
| [46] | British Association of Otolaryngology. Head and Neck Cancer[OL].British: British Association of Otolaryngology,2011. |
| [47] | National Comprehensive Cancer Network. Head and neck cancer[OL].American: National Comprehensive Cancer Network,2012. |
| [48] | Cancer Care Ontario. The role of neoadjuvant chemotherapy in the treatment of locally advanced squamous cell carcinoma of the head and neck (excluding nasopharynx) [OL].Toronto, Ontario: Cancer Care Ontario,2003. |
| [49] | British Columbia Cancer Agency.Head and neck cancer management guidelines[OL].British: British Columbia Cancer Agency,2003. |
| [50] | Clinical Oncological Society of Australia. Evidence Based Guidelines for the Nutritional Management of Patients with Head and Neck Cancer[OL].Australia: Clinical Oncological Society of Australia,2011. |
| [51] | Jack DR, Dawson FR, Reilly JE, et al. Guideline for prophylactic feeding tube insertion in patients undergoing resection of head and neck cancers[J]. J Plast Reconstr Aesthet Surg,2012, 65(5):610-5. |
| [52] | Salama JK, Haddad RI, Kies MS, et al. Clinical practice guidance for radiotherapy planning after induction chemotherapy in locoregionally advanced head-and-neck cancer[J]. Int J Radiat Oncol Biol Phys,2009,75(3):725-33. |
| [53] | Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection[J]. Arch Otolaryngol Head Neck Surg, 2008, 134(5): 536-8. |
| [54] | British Columbia Oral Cancer Prevention Program, BC Cancer Agency; College of Dental Surgeons of British Columbia.Guideline for the early detection of oral cancer in British Columbia 2008[J]. J Can Dent Assoc, 2008,74(3):245. |
| [55] | Renaud-Salis JL,Blanc-Vincent MP,Brugère J,et al. Epidermoid cancers of the oropharnyx[J]. Br J Cancer, 2001,84 Suppl 2:37-41. |
 2014, Vol.41
2014, Vol.41